ISSN 2410-5708 / e-ISSN 2313-7215
Year 9 | No. 26 | p. 257 - 279 | October 2020 - January 2021
© Copyright (2020). National Autonomous University of Nicaragua, Managua.
This document is under a Creative Commons
Attribution-NonCommercial-NoDerivs 4.0 International licence.
Topographic Position Index (TPI) to identify floodable areas and zoning of plant species in a mangrove swamp in the South Pacific of Nicaragua
https://doi.org/10.5377/torreon.v9i26.10264
Submitted on February 6, 2020 / Accepted on July 7, 2020
Ph.D Heyddy Calderón
Ph.D in Hydrology
UNAN-Managua, Geology and Geophysics Institute
MSc. José Enrique Pérez
Master in Enviromental Management
UNAN-Managua
Ph.D Marcel Chow
Ph.D in AstroPhysics Sciences
UNAN-Managua, Geology and Geophysics Institute
Section: Engineering, Industry and Construction
SCIENTIFIC ARTICLES
Keywords: TPI, mangrove forest, geomorphology, flooding areas, integrated management
Abstract
COP7 recognized that mangroves are vulnerable ecosystems and threatened by habitat loss and degradation, which need urgent actions to guarantee their conservation and rational use. Geomorphological and hydrological processes are key to the growth and development of mangrove forests. However, in Central America, very few studies exist in this regard. Mangrove forests offer valuable economic and ecological services, such as tourism and shelter for species of flora and fauna. Furthermore, they protect against sea-level rise caused by climate change. The hydrology of the mangrove swamp of Río Ostional (~ 0.2 km2), in the South Pacific of Nicaragua, has been studied in recent years suggesting a relationship between mangrove species and freshwater inputs. In this project, the use of the Topographic Position Index (TPI) was explored to identify small-scale floodplains with little variation in elevation (~ 3m) in a mangrove swamp. For this, a geomorphological characterization was carried out through a detailed topographic survey of the area and the analysis of the TPI as a method of classifying landforms to identify the flood zones; as well as the zoning of plant species. The classification of the TPI was related to the zoning of species. This relationship will allow the identification of the most sensitive areas to geomorphological modifications of possible anthropic origin, which is an essential input for comprehensive forest management.
Introduction
Central America has about 4,000 km2 of mangroves on the Pacific coast, of which Nicaragua has 400 km2 (Ellison, 2004). The Ostional mangrove forest, located in the municipality of San Juan del Sur, is a typical example of coastal ecosystems in the South Pacific of Nicaragua. The presence of mangroves and sand bars on the beach dominates the estuaries of these rivers (Jiménez, 1992). Ostional beach is also very close to La Flor Wildlife Refuge, and although to a lesser extent, it is also a nesting site for sea turtles. Additionally, the mangrove forest shelters other fauna species (birds, monkeys, small mammals) and is a tourist attraction that generates economic income for the population of Ostional (Calderón, 2014).
The Global Review of Wetland Resources and Priorities for Wetland Inventory (COP7) recognized that mangroves and coral reefs are some of the most vulnerable ecosystems and threatened by loss and degradation of habitats, and consequently, they need urgent and priority actions to guarantee their conservation and rational use. On the other hand, geomorphological and hydrological processes are key for the growth and development of mangrove forests (Feller and Sitnik, 1996; He et al., 2010; Jiménez, 1994,1999; Lugo and Snedaker, 1974, Tomlinson, 1986 ). This system has started to be studied from a hydrological point of view and during this process zoning patterns of mangrove species have been identified in a general way (Calderón, 2015; Calderón et al 2014, De Vries 2016). However, it is necessary to deepen further the understanding between the surface water flows towards the system and its relationship with the zoning of mangrove species. It is necessary to have this knowledge to identify the areas of the mangrove that are most sensitive to morphological changes of natural or anthropogenic origin, such as an alteration to natural relief, the appearance of new gullies, discharge of domestic gray water, or solid waste within the mangrove.
On the other hand, there is no species zoning map, which is essential to generate a comprehensive mangrove management plan. Besides, flood zones are particularly important since the growth and development of the forest depend to a large extent on the delicate balance between freshwater flows and saltwater flows, which also determines the zoning of species. This work aims to contribute to the understanding of the hydrological processes in the mangrove systems of Central America and generate necessary inputs for the Comprehensive Management Plan of the Ostional Mangrove forest. The objective of this project is to test the use of the TPI to classify small-scale floodplains with little variation in elevation (~ 3m) in a mangrove swamp in southwest Nicaragua, to correlate them with zoning patterns of mangrove species.
Study area
Ostional is a small rural coastal community in the municipality of San Juan del Sur, Rivas. It is located in the Holdrige life zone of dry tropical forest. The study area is a floodplain about 0.5 km wide characterized by a blind estuary, closed by a sand bar produced seasonally by the “spring” tide. During the peak of the rainy season (October-November) the flood of the Ostional river breaks the bar releasing the water contained in the estuary (deVries, 2016). The presence of the sand bar helps to maintain a positive water balance during the dry season or drought, which favors the subsistence and growth of mangrove species (Calderón et al., 2014). The soil is clayey and the water table ranges from 1 to 0.5 m during the year. The mangrove species identified in the study area are white, red, piñuela and Buttonwood (deVries 2016).
The local population is around 1,500 people and the economy is based on fishing, tourism, and subsistence agriculture (Calderón, 2014). The mangrove area is one of the tourist attractions offered to visitors for bird watching, mammals, lizards, hiking, and kayaking. However, it is also used as a source of firewood and pasture.
Characterization and zoning of plant species
The survey was carried out in the Ostional estuary making random points trying to give a homogeneous bearing of the place. The study is descriptive and cross-sectional, carried out from October 28 to 30, 2015, enough time to determine the presence and diversity of the plant species existing in the sampled points, given the rapid sampling characteristic of this research. The study population was all the plant species of the forest redoubt, vegetation associated with the mangrove swamp present in detailed transects adjusting to the biotypes of trees, shrubs, and herbs.
A sampling of six 10 x 10 m (100 m2) plots, two for each plant formation, was carried out to obtain homogeneous areas. This method was proposed by Foster et al., (1995), to carry out rapid vegetation evaluations. It was considered that it is usually better to do several small samplings than a few large ones. Foster et al., (1995) mention that 50 individuals for each sample can be an adequate number, with which several representative samplings can be made in a single day (Mateucc and Colma, 1982; Mostacedo & Fredericksen, 2000; Araujo et al ., 2005). The sample was analyzed with biodiversity indices to characterize the mangrove swamp and its associated flora. Margalef indices were used to determine its richness, Shannon-Weinner index to measure diversity, and the Simpson index to assess the dominance of the species. The species were identified with the Flora of Nicaragua bibliography (Steven et al., 2001).
Biodiversity indices
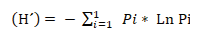
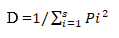
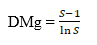
Where:
H´: Shannon diversity index–Wiener
D: Simpson’s dominance index
DMg = Margalef wealth index
Pi: ni / S
Ni: Number of individuals
Pi: Relative abundance of species i (pi: ni / N)
N: total number of individuals
S: specific wealth
Σni: N
Detailed topographic survey
The detailed survey was done with a Leica Ts09 plus total station. The elevations of three piezometers located on the shoreline at the study site were used as benchmarks. Those elevations were surveyed in 2012 with an Ashtech Locus L1 brand differential GPS with 5mm horizontal and 10mm vertical precision. For the data collection, the total station was oriented with the known coordinate method, due to the structure of the mangroves, a prism with a 3 m reach rod was used for high readings and a mini prism of 0.6 m for low readings, the altiplanimetric readings were performed in combination with prism laser and rebound laser measurements. A total of 433 points were raised trying to distribute them evenly in the area. Elevations range from 0.175 masl to 3,104 masl. However, in some places, the local density of trees made it difficult to collect data, so these areas have a lower density of points (Figure 1). The number of topographically surveyed points is conditioned by the density of trees, which prevents the prism from being seen. In some places, with fewer individuals, it was possible to have a higher density of points.
Generation of the Digital Terrain Model (DEM)
The digital elevation model was generated from the data collected in the detailed topographic survey (Figure 1). These data were modeled using the R statistical software. To choose the pixel size, a statistical analysis of the standard deviation of each point was performed with its closest neighbors (five neighbors to ensure statistical robustness). According to the distribution of the standard deviations of these data, a maximum of 2.5 m was obtained, so this value was established as the cell size for the generation of the DEM.
The DEM was generated using simple kriging type interpolation, using the R GSTAT geostatistical library and the data collected from the topographic survey. The Kriging interpolation method is very precise and efficient to other interpolation methods in the creation of digital elevation models, it provides more robust analyzes with statistical foundations (Villatoro, 2008; Vílchez, 2000; Pineda M, 2015), by what this method was used in this study. As mentioned above, a cell size of 2.5 x 2.5 m was chosen. The variogram was adjusted using a spherical model. The selection of other models did not produce significant changes in the DEM.
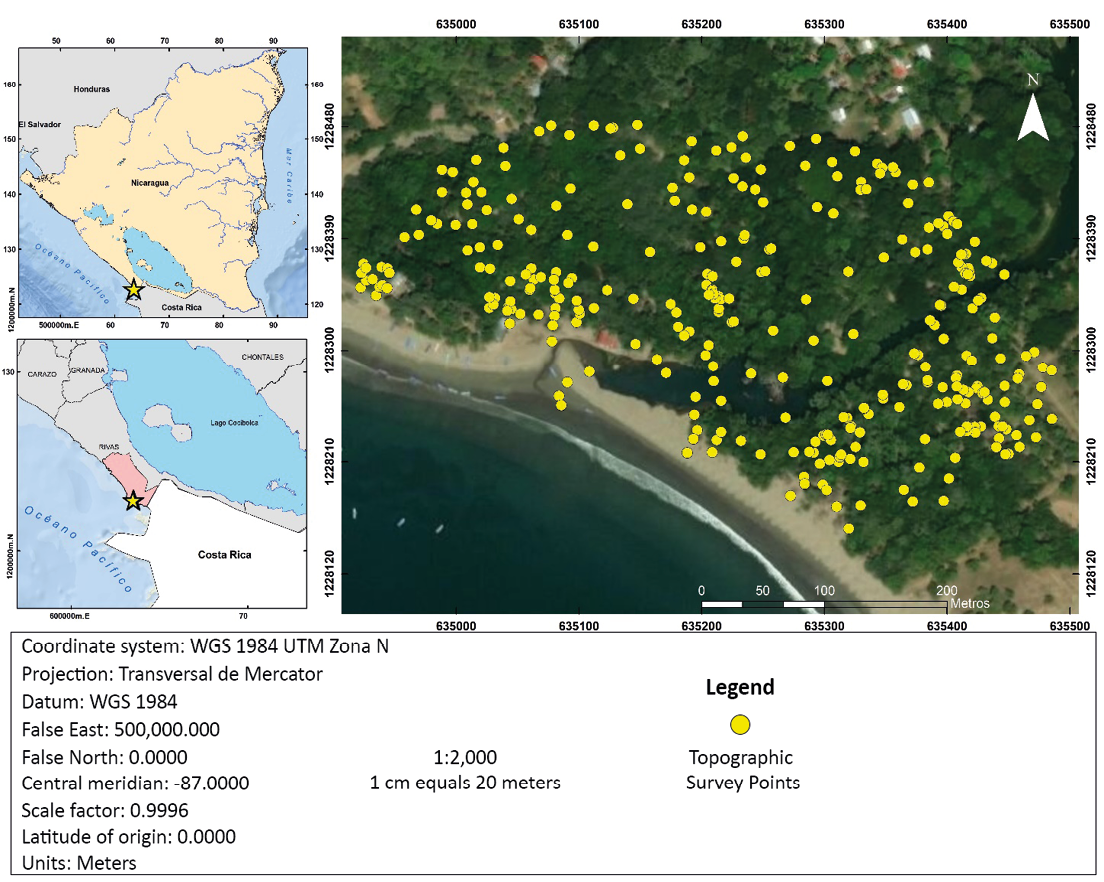
Figure 1. Study area and points of the topographic survey.
Geomorphological classification using TPI
The topographic position index or TPI (Topographic Position Index) allows us to describe morphological aspects of the terrain through the calculation and sectoring of slopes. The TPI is a method of classifying the position of the slope and the types of geomorphology.
The TPI value is given by:
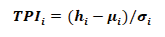
Where the TPIi is calculated for the i-th pixel of the DEM and hi refers to the elevation of said pixel. µi is the average value of all pixels around the i-th pixel within a certain distance range. For this study, a range between 20 m and 50 m was considered, since this scale is consistent with the average length of the valleys and peaks according to the experience gained in the field during the topographic survey. Scales larger than this would cause the loss of the smaller geomorphological units. In the same way, σi is the standard deviation of the DEM pixels within that same range. Therefore the TPI is a statistical comparison of the elevation value of the pixels concerning the pixels in the vicinity. The result of the TPI depends on the scale since it uses values of its vicinity, that is, it depends on the measured lengths and the position of a slope (Weiss, 2001). Positive values represent locations that are higher than their surroundings (Summits). Negative values represent locations that are lower than the surroundings (Valleys). Values close to zero can be flat areas (with a slope close to zero) or areas of constant slope, where a criterion of the angle of the slope can be used to estimate whether it is one or the other case.
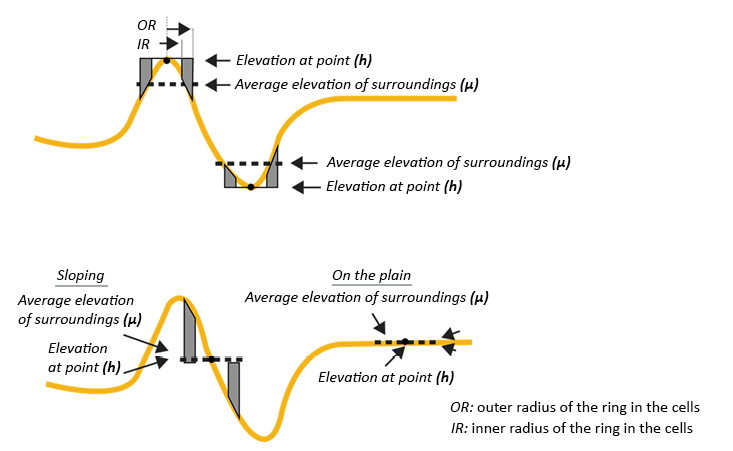
Figure 2. Conceptualization of the TPI (modified from Weiss, 2001)
The TPI generates a map of continuous values. For geomorphological analysis, it is very useful to define thresholds to classify landforms. In this work, the values suggested by Weiss, 2001, which define six landforms, were used as thresholds:
I. Ridges: TPI > 1.0.
II. Upper slope: 1.0 > TPI > 0.5
III. Middle slope: 0.5 > TPI > -0.5 (angles greater than 15°)
IV. Flat: 0.5 > TPI > -0.5 (angles less than 15 °)
V. Lower slope: -0.5 > TPI > -1.0
VI. Valley: TPI < -1.0
Results
Biodiversity indices
The plant formations census, according to the Margalef index, indicates that the site of greatest wealth is the dry forest which surrounds the mangrove swamp and adjacent areas to the river; the lowest value corresponds to the mangrove swamp, where salinity, soil instability, and seasonal floods allow few species to proliferate. According to the Simpson index, which assesses dominance, it indicates the mangrove swamp as dominant, due to the presence of halophilic species (they tolerate salinity), which are the abundant ones, pointing to other plant formations with moderate dominance. The Shannon-Weinner index indicates that the beach and dry forest have almost similar diversity, with the dilemma that species and biotypes are dissimilar. The coastal species are mostly grasses and shrubs that grow on coastal dunes and salinized soils. The dry forest is mostly made up of trees that need deep soils and a neutral pH; finally, the index certifies that the plant diversity in the mangrove swamp is low.
Table 1. Biodiversity Indices
|
Plant formation |
Shannon index (Diversity) |
Simpson index (Dominance) |
Margalef index (Species richness) |
|
Beach |
2.81 |
0.085 |
3.472 |
|
Dry forest |
2.71 |
0.128 |
5.086 |
|
Mangrove swamp |
1.58 |
0.2777 |
1.507 |
Vegetable species
Ostional mangrove
The sampled site consists of 55 census species, located in 25 botanical families. Within the sampling points, 3 marked plant associations were characterized, which are:
1. Coastal vegetation,
2. Mangrove,
3. and dry secondary forest.
The mangrove swamp is characterized by presenting floodable areas with a mixture of fresh and saltwater. These sites are occupied by halophilic species or tolerant to high concentrations of salts and water stress. The species present at this site are Rhizophora mangle L (red), Laguncularia racemosa (L.) C.F. Gaertn (white), Pelliciera Rhizophora Planch. & Triana. (piñuela), Hippomane mancinella L., Prosopis juliflora (Sw.) DC., Conocarpus erectus L. (buttonwood), Coccoloba caracasana Meisn., Bromelia pinguin L., Talipariti tiliaceum (Arruda) Fryxell. These species are characteristic of mangrove zones in the Nicaraguan Pacific, except for Pelliciera Rhizophora, which had not been reported in the Nicaraguan Pacific; but it is known that it is distributed in the Caribbean zone of Nicaragua in Bluefields (Roth 1991). Strangely, this plant has not followed its natural distribution and dispersion since this mangrove is bordered by a ridge of sand, preventing its marine hydro dispersion.
The diversity indexes in the mangroves are low, therefore, in this investigation, we limited ourselves to mentioning the richness of species at the site. The trees found there are quite mature with heights greater than 12 meters with abundant recruit trees that come positioned from the uncovered mangrove areas.

Figure 3. Vegetative species in the Ostional estuary. a) Red mangrove b) Piñuela mangrove c) Coastal vegetation c) Dry forest
Ostional coastal area
It is characterized by having pioneering bushes or latizal species, mainly limited to the presence of marine sand, stones, calcareous residues, and lack of soil. The areas that are exposed to high solar radiation mostly have annual plants with a short life range, these species are limited to the sandbanks. The Shannon index (2.81) shows that the diversity in the tidal areas is moderately high, superior to the mangrove swamp, and compared to the forest and the Simpson index indicates that there is little dominance of the species developed in that area probably due to the ability to be a formation that does not have a plant balance since many species are competing for space.
Dry forest
The dry forest that appears on the banks of the Ostional River presents tree areas with heights greater than 15 meters with a diversity (2.71), according to Shannon less than that of the coastal zone with the great difference that the forest consists of specimens of higher heights, its diversity is less due to the equilibrium pattern of trees that have a long life and are perennial. Regarding the dominant species, there are no results according to the Simpson index that shows a pattern of dominant species in the census populations, keeping the diversity inversely proportional to the dominance reflected in the tables.
Geomorphological classification based on the TPI
As previously noted, the topographic position index is inherently scale-dependent (Weiss, 2001), and the TPI of the same point could change according to its scale. Thus, the TPI with a small analysis size captures the local Peaks and Valleys, while the large analyzes capture characteristics on a large scale (Janness, 2006). Since the terrain is not very large (approximately 300m x 600m), it is preferred to use a small scale (50m radius).
According to this classification, the plain areas surrounded by Summits would be the ones that accumulate the most freshwater (from precipitation or river overflow). The Summits are areas of little accumulation of freshwater, being above the surrounding areas. Steep slope areas are intermediate zones, where not much water accumulation is expected, but surface runoff is expected.
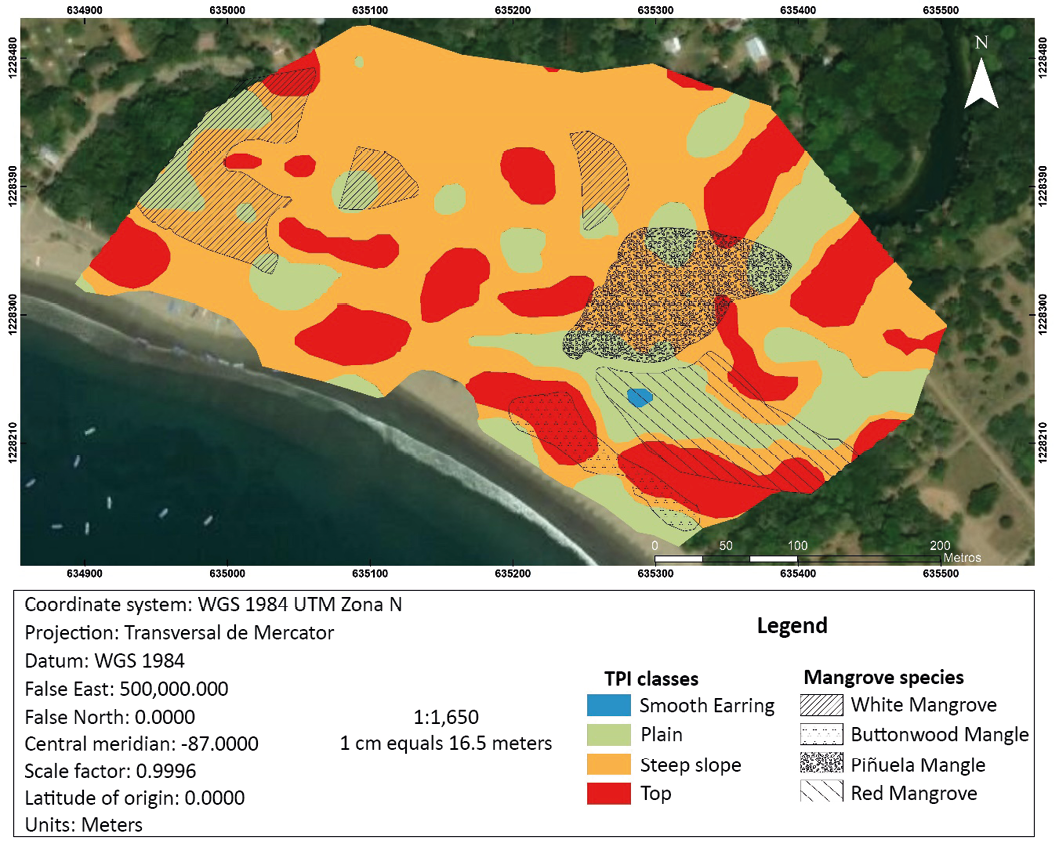
Figure 4. Geomorphological classification and zoning map of Mangrove species
Discussion
Flooding and zoning of mangrove species
The contribution of freshwater to the mangrove system occurs through local precipitation and flooding from the estuary (deVries 2016; Calderón 2014). These contributions of freshwater accumulate thanks to the clay content of the soils in forest areas where there are small depressions or barriers formed by sediment deposits (deVries 2016). In this case, we use the geomorphological classification generated by the TPI as an indicator of areas where due to the slope, more or less accumulation of freshwater is expected.
Zones represented by polygons were defined where one mangrove species is much more abundant than the others (Figure 4). These polygons were superimposed on the landform typologies defined with the TPI to analyze patterns. Outside the polygons, the predominance of a single species could not be established, which limits our analysis.
White mangrove patches are found in the Steep and Flat areas. The piñuela mangrove is in a steep slope area but surrounded by peaks and plains. The red mangrove is concentrated in an area of the Plain, surrounded by Summits. Finally, the button is in the Summits area.
The white mangrove patches are found in the Steep and Flat areas. The piñuela mangrove is in a steep slope area but surrounded by peaks and plains. The red mangrove is concentrated in an area of the Plain, surrounded by Summits. Finally, the button is in the Summits area.
According to Jimenez (1998), in the mangroves of the Central American Pacific with seasonal dry climates, the salinity gradient of the soil increases when moving away from the estuaries and channels, since the frequency of flooding decreases and there is a higher concentration of salts, which is related to less development of tree size. In the case of Ostional, the sand bar closes the system to the sea and flooding occurs either due to the filling of the estuary or due to excessive precipitation. The red mangrove develops in the most floodable areas (near the estuary and the Plains area (flat) surrounded by Summits (ridges). In this case, near the estuary where it has a constant supply of water. Instead, the White Mangrove is concentrated in less floodable areas and the Buttonwood grows on ridges, outside the flood zone, which corresponds to already known zoning patterns (Figure 5).
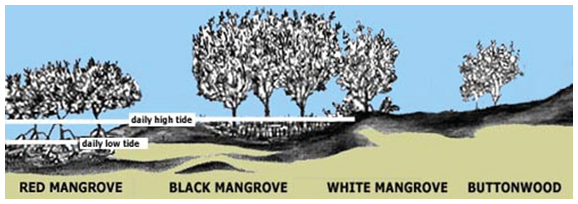
Figure 5. Zoning of mangrove species and flooding areas by the tide (Red mangrove, Black Mangrove, White Mangrove, Buttonwood (https://www.floridamuseum.ufl.edu/southflorida/habitats/mangroves/zonation/)
The presence of piñuela mangrove is associated with the supply of freshwater. In undisturbed environments, it is a minor constituent but tends to spread if other trees have been removed and freshwater is available. This is the case at Ostional. It grows best in wet soils, slowly flooded by high tide (IUCN 2008). This species is on the IUCN Red List of Endangered Species and had not been reported for the Nicaraguan Pacific.
Conclusions
The Ostional estuary forest is made up of a mangrove area that includes: White, Red, Piñuela, and Buttonwood Mangrove. Besides, coastal vegetation and dry tropical forest were identified.
The TPI allowed an adequate classification of landforms in an area with little variation in elevations (~ 3m). The zoning of mangrove species in this area is associated with flood areas (Plains surrounded by Summits): red mangrove. The White Mangrove is located in areas of a steep slope where there are less flooding and the buttonwood in areas of Summits where no flooding occurs.
The piñuela mangrove has been able to spread due to the supply of freshwater from the estuary and the removal of other species due to anthropogenic activity. This species is on the IUCN Red List of Endangered Species and had not been reported for the Nicaraguan Pacific.
Using TPI as a terrain shape classification tool is a useful tool even for small variations in elevation and small scales.
Thanks to
The financing for this work was provided by the Research Fund (FPI) of the UNAN-Managua. The topographic survey was done by the Faculty of Sciences and Engineering with the help of Eng. Roberto Aguirre.
Bibliography
Araujo, A; Bascopé, F; Cardona, V; De la Quintana, D; Fuentes, A; Jørgensen, P; Maldonado, C; Miranda, T; Paniagua, N; & Seidel, R. 2005. Composición florística y estructura del bosque amazónico preandino en el sector del Arroyo Negro, Parque Nacional Madidi, Bolivia; Ecología en Bolivia, Vol. 40(3): 281-303.
Calderon, H., Weeda, R., & Uhlenbrook, S. (2014). Hydrological and geomorphological controls on the water balance components of a mangrove forest during the dry season in the Pacific Coast of Nicaragua. Wetlands, 34(4), 685-697.
De Vries, S. (2016). Blind estuaries during drought: The influences of a sandbar on mangrove trees. Tesis de Maestría. TU Delft.
Ellison, A., Farnsworth, E. & Moore, G. 2010. Pelliciera rhizophorae. The IUCN Red List of Threatened Species 2010: e.T178833A7621318. http://dx.doi.org/10.2305/IUCN.UK.20102.RLTS.T178833A7621318.en
Feller, I & Sitnik, M. 1996. Mangrove ecology: A manual for a field course, A fields manual focused on the biocomplexity on mangrove ecosystem. Smithsonian institution. 1-45.
Foster, B. R., N. C. Hernández, E., E. K. Kakudidi y R. J. Burnham. 1995. Un método de transectos variables para la evaluación rápida de comunidades de plantas en los trópicos. Manuscrito no publicado. Chicago: Environmental and Conservation Programs, Field Museum of Natural History; and Washington, D. C.: Conservation Biology, Conservation International.
He, G., Engel, V., Leonard, L., Croft, A., Childers, D., Laas, M., Deng, Y. and Solo-Gabriele, H. 2010. Factors controlling surface water flow in a low gradient subtropical wetland. Wetlands, 30(2), 275-286.
Jenness, J. 2006. Topographic position index (TPI) v. 1.2. Jenness Enterprises.
Jiménez, J. A., 1992. Mangrove forests of the Pacific coast of Central America. Coastal plant communities of Latin America, 259-267.
Jiménez, J. A., 1994. Los manglares del Pacífico centroamericano (No. 333.91809728 J61). Editorial Fundación UNA.
Jiménez, J. A., Yañez-Arancibia, A. y Lara-Rodriguez, A., 1999. Ambiente, distribucion y caracteristicas estructurales de los manglares del Pacifico de Centro America: contrastes climaticos. En: Yañez-Arancibia, A y Lara-Rodriguez, A. eds. Ecosistemas de manglar en America Tropical. 1era ed. Mexico: Instituto de Ecologia A.C. Centro SEP-CONACYT, 51-70.
Kathiresan, K. 2008. Importance of mangrove ecosystem. Centre of advanced study in marine biology. Annamalai University.1-34.
Lugo, A.E. y Snedaker, S.C. 1974. The ecology of mangroves. Annual Review of Ecology and Sustematics, 5, 39-64.
Matteucc, S y Colma A. 1982. Metodología para el estudio de la vegetación. Departamento de asuntos científicos y tecnológicos de la secretaria general de la organización de los estados americanos. 25. Estado de Falcón, Venezuela.
Mostacedo B & Fredericksen, T. 2000. Manual de métodos básicos de muestreo y análisis en ecología vegetal Editora El País, Bolivia.
Pineda M, M. D. (2015). Evaluación de metodos de interpolación, para generación de modelos digitales de elevacion, en areas planas. Researchgate.
Reyes, M y Tovilla, C.2002. Restauración de áreas alteradas de manglar con Rhizophora mangle en la Costa de Chiapas. Madera y Bosques Número especial, 103-114.
Stevens, W.D., Ulloa, C., Pool, A. y Montiel, O.M. (Eds.). 2001. Flora de Nicaragua. Vol. 85, tomos I, II y III. Missouri Botanical Garden Press, St. Louis Missouri.
Tomlinson, P. 1986. The botany of mangroves. Cambridge University Press, Cambridge, United Kingdom.413.
Vílchez, J. (2000). Evaluación de la exactitud de modelos de elevación digital (MED) de malla regular generados a partir de curvas de nivel. Revista Geográfica Venezolana, 41(2), 239-256.
Villatoro, M. H. (2008). Comparación de los interpoladores IDW y Kriging en la variación espacial de pH, Ca, CICE y P del suelo. Agronomía Costarricense, 32(1), 95-105.
Weiss, A. (2001, July). Topographic position and landforms analysis. In Poster presentation, ESRI user conference, San Diego, CA (Vol. 200).
Appendix
Table 1. Species found in Ostional.
|
Species |
Botanical Family |
|
Annona reticulata L. |
Annonaceae |
|
Cascabela ovata (Cav.) Lippold |
Apocynaceae |
|
Rauvolfia tetraphylla L. |
Apocynaceae |
|
Stemmadenia pubescens Benth. |
Apocynaceae |
|
Crescentia alata Kunth |
Bignoniaceae |
|
Crescentia cujete L. |
Bignoniaceae |
|
Tabebuia rosea (Bertol.) DC. |
Bignoniaceae |
|
Cordia dentata Poir |
Boraginaceae |
|
Varronia inermis (Mill.) Borhidi |
Boraginaceae |
|
Bromelia pinguin L. |
Bromeliaceae |
|
Cleome spinosa Jacq. |
Capparaceae |
|
Conocarpus erectus L. |
Combretaceae |
|
Laguncularia racemosa (L.) C.F. Gaertn. |
Combretaceae |
|
Commelina erecta L. |
Commelinaceae |
|
Ipomoea pes-caprae (L.) R. Br. |
Convolvulaceae |
|
Euphorbia hirta L. |
Euphorbiaceae |
|
Hippomane mancinella L. |
Euphorbiaceae |
|
Hura crepitans L. |
Euphorbiaceae |
|
Jatropha gossypiifolia L. |
Euphorbiaceae |
|
Sapium glandulosum (L.) Morong |
Euphorbiaceae |
|
Canavalia rosea (Sw.) DC. |
Fabaceae |
|
Crotalaria retusa L. |
Fabaceae |
|
Enterolobium cyclocarpum (Jacq.) Griseb |
Fabaceae |
|
Gliricidia sepium (Jacq.) Kunth |
Fabaceae |
|
Haematoxylum brasiletto H. Karst |
Fabaceae |
|
Mimosa pudica L. |
Fabaceae |
|
Pithecellobium lanceolatum (Humb. & Bonpl. ex Willd.) Benth. |
Fabaceae |
|
Prosopis juliflora (Sw.) DC. |
Fabaceae |
|
Samanea saman (Jacq.) Merr. |
Fabaceae |
|
Senna atomaria L. |
Fabaceae |
|
Senna pallida (Vahl) H.S. Irwin & Barneby |
Fabaceae |
|
Tamarindus indica L |
Fabaceae |
|
Vachellia collinsii (Saff.) Seigler & Ebinger |
Fabaceae |
|
Vachellia farnesiana (L.) Wight & Arn. |
Fabaceae |
|
Astraea lobata (L.) Klotzsch |
Malvaceae |
|
Guazuma ulmifolia Lam. |
Malvaceae |
|
Sida sp. |
Malvaceae |
|
Talipariti tiliaceum (Arruda) Fryxell |
Malvaceae |
|
Waltheria indica L. |
Malvaceae |
|
Azadirachta indica A. |
Meliaceae |
|
Trichilia martiana C. DC. |
Meliaceae |
|
Castilla elastica Sessé |
Moraceae |
|
Pisonia macranthocarpa (Donn. Sm.) Donn. Sm. |
Nyctaginaceae |
|
Passiflora foetida L. |
Passifloraceae |
|
Turnera scabra Millsp |
Passifloraceae |
|
Petiveria alliacea L. |
Phytolaccaceae |
|
Piper sp. |
Piperaceae |
|
Coccoloba caracasana Meisn. |
Polygonaceae |
|
Coccoloba floribunda (Benth.) Lindau |
Polygonaceae |
|
Rhizophora mangle L. |
Rhizophoraceae |
|
Thouinidium decandrum (Bonpl.) Radlk. |
Sapindaceae |
|
Simarouba amara Aubl. |
Simaroubaceae |
|
Pelliciera rhizophorae Planch. & Triana |
Tetrameristaceae |
|
Cissus verticillata (L.) Nicolson & C.E. Jarvis |
Vitaceae |
Mangrove
|
Species |
Family |
|
Rhizophora mangle L. |
Rhizophoraceae |
|
Laguncularia racemosa (L.) C.F. Gaertn. |
Combretaceae |
|
Pelliciera rhizophorae Planch. & Triana |
Tetrameristaceae |
|
Hippomane mancinella L. |
Euphorbiaceae |
|
Prosopis juliflora (Sw.) DC. |
Fabaceae |
|
Conocarpus erectus L. |
Combretaceae |
|
Coccoloba caracasana Meisn. |
Polygonaceae |
|
Bromelia pinguin L. |
Bromeliaceae |
|
Talipariti tiliaceum (Arruda) Fryxell |
Malvaceae |
Table 2. With the application of the diversity index present in the coastal area of the beaches of Ostional.
|
Species |
ni |
Pi |
Ln (Pi) |
H´ |
|
Astraea lobata (L.) Klotzsch |
2 |
0,008403361 |
-4,779123493 |
-0,040160702 |
|
Azadirachta indica A. |
19 |
0,079831933 |
-2,527831695 |
-0,20180169 |
|
Crescentia cujete L. |
3 |
0,012605042 |
-4,373658385 |
-0,055130148 |
|
Canavalia rosea (Sw.) DC. |
6 |
0,025210084 |
-3,680511204 |
-0,092785997 |
|
Cascabela ovata (Cav.) Lippold |
2 |
0,008403361 |
-4,779123493 |
-0,040160702 |
|
Cissus verticillata (L.) Nicolson & C.E. Jarvis |
1 |
0,004201681 |
-5,472270674 |
-0,022992734 |
|
Cleome spinosa Jacq. |
4 |
0,016806723 |
-4,085976313 |
-0,068671871 |
|
Commelina erecta L. |
4 |
0,016806723 |
-4,085976313 |
-0,068671871 |
|
Crotalaria retusa L. |
2 |
0,008403361 |
-4,779123493 |
-0,040160702 |
|
Euphorbia hirta L. |
16 |
0,067226891 |
-2,699681951 |
-0,181491224 |
|
Guazuma ulmifolia Lam. |
5 |
0,021008403 |
-3,862832761 |
-0,081151949 |
|
Haematoxylum brasiletto H. Karst |
10 |
0,042016807 |
-3,169685581 |
-0,133180066 |
|
Hippomane mancinella L. |
1 |
0,004201681 |
-5,472270674 |
-0,022992734 |
|
Ipomoea pes-caprae (L.) R. Br. |
10 |
0,042016807 |
-3,169685581 |
-0,133180066 |
|
Jatropha gossypiifolia L. |
26 |
0,109243697 |
-2,214174136 |
-0,241884569 |
|
Mimosa pudica L. |
3 |
0,012605042 |
-4,373658385 |
-0,055130148 |
|
Passiflora foetida L. |
2 |
0,008403361 |
-4,779123493 |
-0,040160702 |
|
Petiveria alliacea L. |
2 |
0,008403361 |
-4,779123493 |
-0,040160702 |
|
Rauvolfia tetraphylla L. |
3 |
0,012605042 |
-4,373658385 |
-0,055130148 |
|
Senna pallida (Vahl) H.S. Irwin & Barneby |
23 |
0,096638655 |
-2,336776458 |
-0,225822935 |
|
Sida sp. |
9 |
0,037815126 |
-3,275046096 |
-0,123846281 |
|
Simarouba amara Aubl. |
2 |
0,008403361 |
-4,779123493 |
-0,040160702 |
|
Stemmadenia pubescens Benth. |
3 |
0,012605042 |
-4,373658385 |
-0,055130148 |
|
Tamarindus indica L |
4 |
0,016806723 |
-4,085976313 |
-0,068671871 |
|
Turnera scabra Millsp |
22 |
0,092436975 |
-2,38122822 |
-0,220113533 |
|
Vachellia collinsii (Saff.) Seigler & Ebinger |
45 |
0,18907563 |
-1,665608184 |
-0,314925917 |
|
Varronia inermis (Mill.) Borhidi |
5 |
0,021008403 |
-3,862832761 |
-0,081151949 |
|
Waltheria indica L. |
4 |
0,016806723 |
-4,085976313 |
-0,068671871 |
|
238 |
-2,813493928 |
Table 3. Explaination of the Shannon index for diversity in the dry forest present near the Ostional.
|
Species |
ni |
pi |
Ln(pi) |
H´ |
|
Annona reticulata L. |
2 |
0,0645161 |
-2,74084 |
-0,176828 |
|
Azadirachta indica A. |
31 |
0,2695652 |
-1,310945 |
-0,353385 |
|
Castilla elastica Sessé |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Cissus verticillata (L.) Nicolson & C.E. Jarvis |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Coccoloba caracasana Meisn. |
2 |
0,0173913 |
-4,051785 |
-0,070466 |
|
Coccoloba floribunda (Benth.) Lindau |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Cordia dentata Poir |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Crescentia alata Kunth |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Crescentia cujete L. |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Enterolobium cyclocarpum (Jacq.) Griseb |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Gliricidia sepium (Jacq.) Kunth |
2 |
0,0173913 |
-4,051785 |
-0,070466 |
|
Guazuma ulmifolia Lam. |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Hura crepitans L. |
3 |
0,026087 |
-3,64632 |
-0,095121 |
|
Piper sp. |
2 |
0,0173913 |
-4,051785 |
-0,070466 |
|
Pisonia macranthocarpa (Donn. Sm.) Donn. Sm. |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Pithecellobium lanceolatum (Humb. & Bonpl. ex Willd.) Benth. |
4 |
0,0347826 |
-3,358638 |
-0,116822 |
|
Prosopis juliflora (Sw.) DC. |
5 |
0,0434783 |
-3,135494 |
-0,136326 |
|
Samanea saman (Jacq.) Merr. |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Sapium glandulosum (L.) Morong |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Senna atomaria L. |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Simarouba amara Aubl. |
2 |
0,0173913 |
-4,051785 |
-0,070466 |
|
Stemmadenia pubescens Benth. |
4 |
0,0347826 |
-3,358638 |
-0,116822 |
|
Tabebuia rosea (Bertol.) DC. |
1 |
0,0086957 |
-4,744932 |
-0,04126 |
|
Thouinidium decandrum (Bonpl.) Radlk. |
12 |
0,1043478 |
-2,260025 |
-0,235829 |
|
Trichilia martiana C. DC. |
11 |
0,0956522 |
-2,347037 |
-0,224499 |
|
Vachellia collinsii (Saff.) Seigler & Ebinger |
14 |
0,1217391 |
-2,105875 |
-0,256367 |
|
Vachellia farnesiana (L.) Wight & Arn. |
8 |
0,0695652 |
-2,665491 |
-0,185425 |
|
115 |
-2,715673 |
Simpson Index that demonstrates and supports diversity in the area where dominant plants do not exist in the census conducted in the coastal area.
|
Species |
ni |
Pi |
Pi ^2 |
|
Astraea lobata (L.) Klotzsch |
2 |
0,008403361 |
7,06165E-05 |
|
Azadirachta indica A. |
19 |
0,079831933 |
0,006373137 |
|
Crescentia cujete L. |
3 |
0,012605042 |
0,000158887 |
|
Canavalia rosea (Sw.) DC. |
6 |
0,025210084 |
0,000635548 |
|
Cascabela ovata (Cav.) Lippold |
2 |
0,008403361 |
7,06165E-05 |
|
Cissus verticillata (L.) Nicolson & C.E. Jarvis |
1 |
0,004201681 |
1,76541E-05 |
|
Cleome spinosa Jacq. |
4 |
0,016806723 |
0,000282466 |
|
Commelina erecta L. |
4 |
0,016806723 |
0,000282466 |
|
Crotalaria retusa L. |
2 |
0,008403361 |
7,06165E-05 |
|
Euphorbia hirta L. |
16 |
0,067226891 |
0,004519455 |
|
Guazuma ulmifolia Lam. |
5 |
0,021008403 |
0,000441353 |
|
Haematoxylum brasiletto H. Karst |
10 |
0,042016807 |
0,001765412 |
|
Hippomane mancinella L. |
1 |
0,004201681 |
1,76541E-05 |
|
Ipomoea pes-caprae (L.) R. Br. |
10 |
0,042016807 |
0,001765412 |
|
Jatropha gossypiifolia L. |
26 |
0,109243697 |
0,011934185 |
|
Mimosa pudica L. |
3 |
0,012605042 |
0,000158887 |
|
Passiflora foetida L. |
2 |
0,008403361 |
7,06165E-05 |
|
Petiveria alliacea L. |
2 |
0,008403361 |
7,06165E-05 |
|
Rauvolfia tetraphylla L. |
3 |
0,012605042 |
0,000158887 |
|
Senna pallida (Vahl) H.S. Irwin & Barneby |
23 |
0,096638655 |
0,00933903 |
|
Sida sp. |
9 |
0,037815126 |
0,001429984 |
|
Simarouba amara Aubl. |
2 |
0,008403361 |
7,06165E-05 |
|
Stemmadenia pubescens Benth. |
3 |
0,012605042 |
0,000158887 |
|
Tamarindus indica L |
4 |
0,016806723 |
0,000282466 |
|
Turnera scabra Millsp |
22 |
0,092436975 |
0,008544594 |
|
Vachellia collinsii (Saff.) Seigler & Ebinger |
45 |
0,18907563 |
0,035749594 |
|
Varronia inermis (Mill.) Borhidi |
5 |
0,021008403 |
0,000441353 |
|
Waltheria indica L. |
4 |
0,016806723 |
0,000282466 |
|
238 |
D |
0,085163477 |
|
|
1-D |
0,914836523 |
Simpson's index in the dry forest where it is shown that it is in condition of balanced diversity or in search of balance where pioneer species are disappearing to remain perennial.
|
Species |
ni |
pi |
Pi ^2 |
|
Annona reticulata L. |
2 |
0,064516129 |
0,0041623 |
|
Azadirachta indica A. |
31 |
0,269565217 |
0,0726654 |
|
Castilla elastica Sessé |
1 |
0,008695652 |
7,561E-05 |
|
Cissus verticillata (L.) Nicolson & C.E. Jarvis |
1 |
0,008695652 |
7,561E-05 |
|
Coccoloba caracasana Meisn. |
2 |
0,017391304 |
0,0003025 |
|
Coccoloba floribunda (Benth.) Lindau |
1 |
0,008695652 |
7,561E-05 |
|
Cordia dentata Poir |
1 |
0,008695652 |
7,561E-05 |
|
Crescentia alata Kunth |
1 |
0,008695652 |
7,561E-05 |
|
Crescentia cujete L. |
1 |
0,008695652 |
7,561E-05 |
|
Enterolobium cyclocarpum (Jacq.) Griseb |
1 |
0,008695652 |
7,561E-05 |
|
Gliricidia sepium (Jacq.) Kunth |
2 |
0,017391304 |
0,0003025 |
|
Guazuma ulmifolia Lam. |
1 |
0,008695652 |
7,561E-05 |
|
Hura crepitans L. |
3 |
0,026086957 |
0,0006805 |
|
Piper sp. |
2 |
0,017391304 |
0,0003025 |
|
Pisonia macranthocarpa (Donn. Sm.) Donn. Sm. |
1 |
0,008695652 |
7,561E-05 |
|
Pithecellobium lanceolatum (Humb. & ) |
4 |
0,034782609 |
0,0012098 |
|
Prosopis juliflora (Sw.) DC. |
5 |
0,043478261 |
0,0018904 |
|
Samanea saman (Jacq.) Merr. |
1 |
0,008695652 |
7,561E-05 |
|
Sapium glandulosum (L.) Morong |
1 |
0,008695652 |
7,561E-05 |
|
Senna atomaria L. |
1 |
0,008695652 |
7,561E-05 |
|
Simarouba amara Aubl. |
2 |
0,017391304 |
0,0003025 |
|
Stemmadenia pubescens Benth. |
4 |
0,034782609 |
0,0012098 |
|
Tabebuia rosea (Bertol.) DC. |
1 |
0,008695652 |
7,561E-05 |
|
Thouinidium decandrum (Bonpl.) Radlk. |
12 |
0,104347826 |
0,0108885 |
|
Trichilia martiana C. DC. |
11 |
0,095652174 |
0,0091493 |
|
Vachellia collinsii (Saff.) Seigler & Ebinger |
14 |
0,12173913 |
0,0148204 |
|
Vachellia farnesiana (L.) Wight & Arn. |
8 |
0,069565217 |
0,0048393 |
|
115 |
D |
0,1237086 |
|
|
1-D |
0,8762914 |