ISSN 2410-5708 / e-ISSN 2313-7215
Year 10 | No. 29 | October 2021- January 2022
© Copyright (2021). National Autonomous University of Nicaragua, Managua.
This document is under a Creative Commons
Attribution-NonCommercial-NoDerivs 4.0 International licence.
Microbial degradation of pesticide residues in bioremediation devices type biological bed
https://doi.org/10.5377/rtu.v10i29.12741
Submitted on July 14, 2020 / Accepted on June 22, 2021
B.A.Sc. Mariano Guerrero
Bachelor in Chemistry
National Autonomous University of Nicaragua, Managua
Center for Aquatic Resources Research of Nicaragua, CIRA
Laboratory of Organic Contaminants
mariano.guerrero@cira.unan.edu.ni
https://orcid.org/0000-0001-7239-908X
M.A. Josseth Díaz
Master in Environmental Management
National Autonomous University of Nicaragua, Managua
Center for Aquatic Resources Research of Nicaragua, CIRA
Laboratory of Organic Contaminants
Section: Sciences
Scientific Articles
Keywords: Biological substrate, cypermethrin, degradation, point contamination, ligninolytic fungi.
Abstract
The improper handling of agrochemical residues at agricultural production sites implies a potential risk of contamination of surface and groundwater. In these places there are specific sources of pollution that, by leaching or runoff, deposit contaminants in the bodies of water adjacent to the cultivation areas. To reduce this risk in some areas biological beds are used, this is a biologically active matrix that retains and accelerates the degradation process of pesticides through ligninolytic fungi that develop in the lignocellulosic material that makes up the biological bed.
In this study the ability of two biological substrates to degrade and retain cypermethrin, one made with sorghum straw and the other using coconut husk as lignocellulosic material, were evaluated, the experiments carried out reflect that the substrate made from sorghum straw has a higher efficiency in terms of retention and degradation of cypermethrin achieving an average retention of 37% of the aggregate mixture and a degradation of 90% of cypermethrin was estimated over a period of thirty days.
1. Introduction
Improper handling of pesticides can contaminate ecosystems, especially groundwater and surface water. Several studies have shown that point sources are the major contributors to pollution (Castillo, Torstensson, & Stenström, 2008). An example of a point source of contamination is the site filling and washing of spray equipment. The frequency and widespread use of man-made chemicals have led to a remarkable effort to implement new technologies to reduce or eliminate these pollutants from the environment (Meleiro, Zelayrán, Consiglio, & Nitschke, 2011).
An alternative to reduce these impacts is the use of biobeds or biobeds at pesticide mixture preparation sites, a simple and inexpensive construction designed to collect and degrade pesticide spills. The purpose is that pesticide handling during filling and washing of spraying equipment and pesticide containers is done on top of the biobed so that if accidental spills occur, the pesticides can be retained and degraded in the biobed. (Castillo, Torstensson, & Stenström, 2008).
The fate of pesticides in the environment is affected by microbial activity, some of them are easily degraded by microorganisms (Aislabie & Lloyd-Jones, 1995), The degradation of these in a biological bed is achieved thanks to the microbial activity provided by the biological substrate by stimulating the growth of white-rot fungi with the ability to degrade lignin through the formation of ligninolytic enzymes, such as peroxidases and laccases (phenoloxidase activity), which have also been shown to be efficient in the degradation of pesticides (Project D09R1006, 2013).
Cypermethrin and its metabolite 3-phenoxybenzoic acid (PBA) have exerted adverse biological effects in the environment; therefore, it is of vital importance to develop different methods to improve their degradation (XIE, ZHOU, WANG, & CHEN, 2008). In this study, the ability of three biological substrates to degrade and retain cypermethrin was estimated. Experiments were conducted at the laboratory and pilot scales to establish which of them could be used as an alternative to improve the management of pesticide residues in the preparation of mixtures, washing of spraying equipment, and packaging of cypermethrin, frequently used by farmers in different regions of Nicaragua to control insect pests.
This study evaluates the capacity of a biological substrate rich in lignin to degrade cypermethrin and was financed thanks to the funds for research projects (FPI) of the UNAN -Managua, under project number 17201604.
2. Methodology
Pesticides (herbicides, fungicides, insecticides) are widely used in agriculture and industry worldwide. More than 55% of the land used for agricultural production in developing countries uses about 26% of the total pesticides produced in the world (Mendoza, Perea, Salvador, Morales, & Perez, 2011). This is an indication that, in agricultural areas, especially where there are non-technified farming systems, there may be excessive use of agrochemicals.
Cypermethrin is an insecticide-type pesticide widely used in the control of insect pests and is routinely used at the agricultural level, for transboundary pest control, and sometimes at the household level. Cypermethrin adsorbs very strongly on soil particles, especially in soils containing large amounts of clay or organic matter. (World Health Organization, 1989)
In an attempt to manage agrochemical residues, pilot-scale experiments were conducted to evaluate the capacity of two biological substrates (Biomix) to retain a cypermethrin mixture prepared at a concentration 5 times higher (1000 mg/l) than that recommended by the manufacturer (200 mg/l). The substrate with the best retention was then selected and the degradation rate of cypermethrin was estimated in laboratory-scale experiments. This experiment was conducted with high cypermethrin concentrations since it is assumed that the bio-mixture will be used to handle residues from washing empty containers, spraying equipment, and preparing the cypermethrin mixture.
2.1. Components and preparation of the bio-mixture
The substrates evaluated in the experiments were prepared from soil, poultry manure, and organic material with a high lignin content; this organic material was the variant in the experiments, using coconut husks and sorghum straw
Soil: Soil corresponds to 25% of the mixture, and is extracted from the first 20 centimeters of depth, not including the topsoil. Soil contributes various microorganisms to the biological mixture, which actively participate in the degradation of pesticides. Likewise, the soil has a great capacity to retain pesticides, depending on its characteristics such as pH and organic matter content (Aparicio et al., 2015), (Aparicio, et al., 2015).
Chicken manure: In the case of chicken manure used as compost, i.e., as organic fertilizer, it is necessary to ferment chicken excrement to transform the chemicals it contains, such as phosphorus, potassium, nitrogen, and carbon. This makes up 25% of the biomix and provides the nutrients necessary for the growth of lignin decomposing microorganisms.
Organic material: This is a material with high lignin content that is obtained in the field mainly as crop residues. For this experiment, coconut husk and sorghum straw were used, which contains 18 % lignin (Prinsen, 2013). These are very important components of the biomix as they allow or favor the development of many microorganisms, especially white-rot fungi which have a high capacity to degrade pesticides. This component makes up 50% of the biological substrate.
Table 1.
Components of the biological mixture
|
Components of the Biomix (%) |
||
|
Material |
Biomixture 1 |
Biomixture 2 |
|
Soil |
25 |
25 |
|
Poultry manure |
25 |
25 |
|
Coconut husk |
50 |
- |
|
Sorghum straw |
50 |
|
The biomixture was prepared in the portions described in Table 1. Each of the organic materials was crushed and homogenized with the rest of the components, and water was added until a humidity percentage of approximately 50-60% was obtained and the biomix was left to stabilize and mature for a period of 2 months. The soil and poultry manure used in all experiments was collected from the same site and homogenized so that the proportions and type of this material were the same in all experiments.
2.2. Retention of the cypermethrin mixture
Pilot-scale experiments were carried out to estimate the cypermethrin retention capacity of the bio-mixtures made from coconut husk and sorghum straw; each of these experiments was carried out in duplicate to identify variations in the results.
An approximate volume of 20.00 dm3 of biomixture was deposited in a plastic container, through which 10.00 liters of cypermethrin mixture were filtered over a period of 24 hours with an approximate outflow of 14.00 ml/min. This stage was carried out continuously for 5 days to evaluate the saturation of the substrate. An aliquot of approximately 100.00 ml of the filtrate obtained was collected for subsequent analysis in the laboratory.
2.3. Degradation of cypermethrin
This experiment was carried out at laboratory scale. The degradation of cypermethrin in the biological mixture made from sorghum straw was evaluated during a period of 30 days (0, 5, 10, 15, 20, 25, 30 days). 14 samples were prepared with approximately 20.00 g of bio-mixture using sorghum straw as organic material and enriched with 2.00 ml of the mixture of 1000.00 mg/l to obtain a concentration of cypermethrin in the substrate of 100.00 mg/kg. The analysis was performed in duplicate and the samples to be processed in each period were selected from the batch of samples at random.
2.4. Sample processing
The liquid and solid samples were extracted with dichloromethane according to the procedure described by the CIRA/UNAN-Managua organic contaminants laboratory for each of these matrices.
In the liquid samples, an aliquot of 1.00 ml of the total collected was measured, deposited in a 1000.00 ml balloon, and gauged up to the mark of enrace. The total content of the balloon was extracted with 180.00 ml of dichloromethane (3 x 60.00 ml), the organic extract was filtered with anhydrous sodium sulfate and deposited in a 250.00 ml balloon and then reconcentrated with a rotary evaporator (BÜCHI RE 121) to a volume of approximately 3.00 ml, with the change of solvent to hexane. The content of the balloon was transferred to a tube of 12.00 ml, it was reconcentrated with a current of inert N2 to an approximate volume of 2.00 ml to then make cleaning of sulfur with 1.00 ml of 2 propanol + 1.00 ml TBA 0.1 M (tetrabutylammonium hydrogen sulfate) saturated with sodium sulfite + 100.00 mg of sodium sulfite + 4.00 ml of extracted water. The obtained organic extract was made up to 1.00 ml and 1 µl was injected into the gas chromatograph with electron capture detector (GC-ECD).
For the solid samples, a total of 20.00 g was weighed and extracted with 60.00 ml dichloromethane (3 x 20.00 ml) by vortex stirring (Heidolph REAX 2000 vortex mixer). The organic extract was filtered with anhydrous sodium sulfate and deposited in a 250.00 ml balloon and then reconcentrated with a rotary evaporator (BÜCHI RE 121) to a volume of approximately 3.00 ml, with the change of solvent to hexane. The content of the balloon was transferred to a 12.00 ml tube, reconcentrated with a current of inert N2 to an approximate volume of 2.00 ml to then carry out sulfur cleaning. The obtained organic extract was made up to 1.00 ml and 1 µl was injected into the gas chromatograph with electron capture detector (GC-ECD).
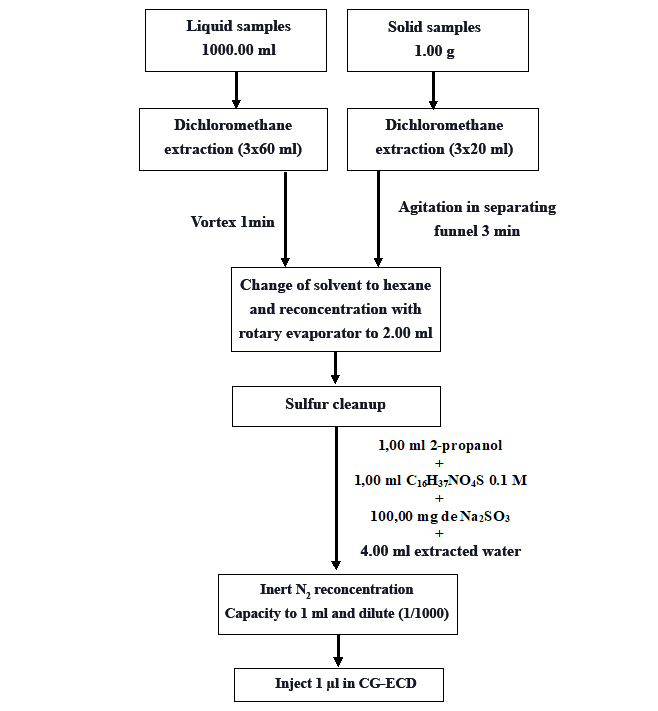
Figure 1.
Pyrethroid Sample Extraction
2.5. Instrumental conditions for sample analysis
Sample extracts were injected into a gas chromatograph with electron capture detector (Perkin Elmer Clarus 500). Agilent DB-5 column of (30 m x 0.32 mm) 0.25 micrometer stationary phase film thickness. Oven programming 100°C hold time 1 minute, 4°C/min up to 200°C, 3°C/min up to 230°C, 15°C/min up to 280°C, hold time 5 minutes; Injector temperature 250°C; Injection volume 1 µl; Carrier gas hydrogen with flow rate 2 ml/min; Detector temperature 350 °C; Backup gas nitrogen with flow rate 28 ml/min; Range 1 and attenuation -4.
3. Results
The biological substrates elaborated based on sorghum straw (Biomix 2) and coconut husk (Biomix 1) reflect results of retention in average during the 5 days of experiment were 86.39 % and 66.07% respectively, however, the experiment carried out with coconut husk reflects a greater coefficient of variation and a decrease in the removal of 61.78 % when comparing the first and the last day, while with sorghum straw the decrease was 28.34 %.
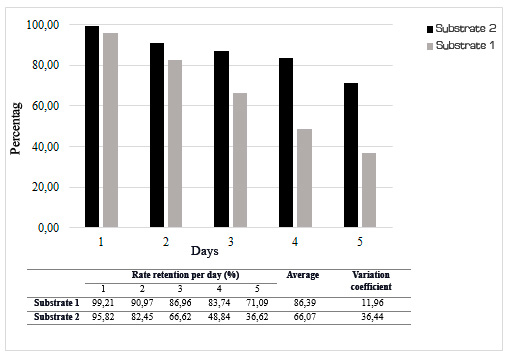
Figure 2.
Cypermethrin retention in the biomixture
These results (Figure 2) reflect greater efficiency in the retention of cypermethrin for the experiments using sorghum straw as a biological substrate, for this reason, it was decided to use this material to evaluate the degradation of cypermethrin using this material to provide lignin to the biomixture. The initial concentration of cypermethrin in the biomixture was approximately 100.00 mg/kg at the end of the 30-day experiment, a final average concentration of cypermethrin of 11.35 mg/kg was obtained.
Table 2.
Concentration of cypermethrin in the samples.
|
Days |
Concentration mg/kg |
Percentage difference (%) |
||
|
Sample |
Replicate |
Average |
||
|
0 |
100,00 |
100,00 |
100,00 |
0,00 |
|
5 |
75,60 |
74,20 |
74,90 |
1,87 |
|
10 |
50,00 |
50,10 |
50,05 |
0,20 |
|
15 |
40,10 |
43,30 |
41,70 |
7,67 |
|
20 |
30,50 |
35,20 |
32,85 |
14,31 |
|
25 |
13,50 |
15,20 |
14,35 |
11,85 |
|
30 |
10,20 |
12,50 |
11,35 |
20,26 |
When evaluating the degradation of cypermethrin in the experiment based on sorghum straw (Figure 3), it was determined that 90% of the cypermethrin present in the biomix was degraded in a period of 30 days. The data obtained show an exponential distribution with a coefficient of determination (r2) of 0.96.
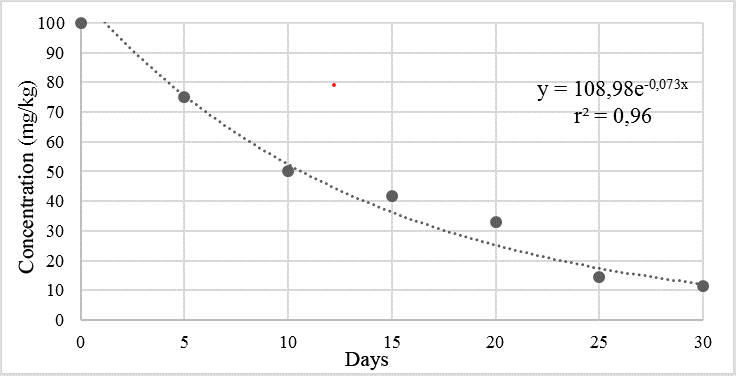
Figure 3.
Degradation of cypermethrin in biomixture 2.
4. Discussion
The biomixture prepared with sorghum straw as organic material to provide lignin to the substrate shows better results in cypermethrin retention, achieving 86 % retention of the cypermethrin added to the substrate during the 5 days of the experiment, which shows that the substrate is efficient for retaining cypermethrin spills and managing the residues from washing and preparation of mixtures.
It was determined that 90% of the cypermethrin added to the samples degraded in a period of 30 days and even though high volumes of liquids were poured at mixture concentrations above the manufacturer’s recommendations for crop spraying, the substrate made from sorghum straw allowed accelerating the degradation process of cypermethrin, however, the simple removal of the original pesticide molecule does not indicate that the treatment is environmentally friendly due to the formation of highly toxic metabolites that are difficult to identify and quantify (Rodriguez, Castro, & Lizano, 2018).
To determine if there is any type of degradation metabolite in the degradation process the study must be expanded and identify these molecules, however, the function of the biological bed for spill retention manages to avoid the entrainment or leaching of pesticide residues towards some water body or the aquifer.
5. Conclusions
The experiments carried out in the retention and degradation tests show that the biological mixture made from sorghum straw is effective for the management of cypermethrin residues and could be used to discharge the liquid residues from the washing of spraying equipment and rinsing of empty pesticide containers and thus accelerate the natural degradation process of this pesticide. It is advisable to evaluate the efficiency of the substrate in the field and demonstrate its effectiveness in retaining and degrading pesticides for a longer period, under real working conditions, and thus identify which factors could influence the efficiency of the substrate. It is also important to validate the methodology with other pesticide molecules and determine if there could be variations in degradation when using it with mixtures of pesticides and especially when some type of fungicide is added to the mixture.
6. ACKNOWLEDGEMENTS
This study was financed thanks to the Funds for Research Projects (FPI) of the UNAN-Managua, under project number 17201604.
References
Aislabie, J., & Lloyd-Jones, G. (1955). A review of bacterial degradation of pesticides. Australian journal of soil research, 925-942.
Aparicio, V., De Gerónimo, E., Hernández, K., Pérez, D., Portocarrero, R., & Vidal, C. (2015). Los plaguicidas agregados al suelo y su destino en el ambiente. INTA.
Castillo, M., Torstensson, L., & Stenström, J. (2008). Camas Biológicas Para La Preservación Del Medio Ambiente De La Contaminación Por Pesticidas. Journal of Agricultural and Food Chemistry 56, 6206-6219.
Meleiro, A., Zelayrán, G., Consiglio, M., & Nitschke, M. (2011). Biodegradation of Pesticides. Pesticides in the Modern World – Pesticides Use and Management, 407-438
Mendoza, J., Perea, Y., Salvador, J., Morales, J., & Pérez, G. (2011). Biodegradación bacteriana de plaguicidas permetrina y cipermetrina en cultivo lote. Avances en ciencia e ingeniería, 45-55.
Prinsen, P. (2013). Caracterización química y estructural de lignina y lípidos de materiales lignocelulósicos de interés industrial (Memoria de investigación para optar al grado de doctor en ciencias químicas).
Proyecto D09R1006. (2013). Manual de construcción y operación de lechos biológicos. Universidad de La Frontera.
Rodríguez, C., Castro, V., & Lizano, V. (2018). Alternative approaches to determine the efficiency of biomixtures used for pesticide degradation in biopurification systems. En E. Dino, & R. Nallin, Toxicity and biodegradation testing (págs. 57-73)
World Health Organization. (1989). Environmental Health Criteria. Cypermethrin. Ginebra.
Xie, W.-J., Zhou, J.-M., Wang, H.-Y., & Chen, X.-Q. (2008). Effect of nitrogen on the degradation of cypermethrin and its metabolite 3-phenoxybenzoic acid in soil. Pedosphere, 638–644.