ISSN 2410-5708 / e-ISSN 2313-7215
Year 9 | No. 24 | p. 120-137 | February - May 2020
© Copyright (2020). National Autonomous University of Nicaragua, Managua.
This document is under a Creative Commons
Attribution-NonCommercial-NoDerivs 4.0 International licence.
Analysis of the proximal composition and insecticidal potential of the soursop seed (Annona muricata L.) for the control of the corn cogollero worm (Spodoptera frugiperda J. E. Smith)
https://doi.org/10.5377/torreon.v9i24.9722
Submitted on February 13th, 2020 / Accepted on February 19th, 2020
B.A. María Guerra Blandino
Thesis specialists
Biotechnology Laboratory, UNAN-Managua
B.A. José Poveda Suárez
Thesis specialists
Biotechnology Laboratory, UNAN-Managua
M.Sc. Samantha Miranda Calero
Researcher
Biotechnology Laboratory, UNAN-Managua
Ph.D. Martha Lacayo Romero
Researcher
Biotechnology Laboratory, UNAN-Managua
Ph.D. Fátima Bolaños Ortega
Researcher
Nicaraguan Institute of Agricultural Technology (NIAT)
Eng. Henry Pedroza Chamorro
Researcher
Nicaraguan Institute of Agricultural Technology (NIAT)
Keywords: Annona muricata L., insecticide potential, proximate composition, seed, soursop.
ABSTRACT
Soursop (Annona muricata L.) is a tropical species with a growing global interest due to its multiple properties, and its applications in health and agriculture fields. In order to contribute with the knowledge of the available acquis and promote its use to solve national problems, this research analyzed the proximal composition and the insecticidal potential of soursop seeds on the cogollero worm (Spodoptera frugiperda JE Smith), a Lepidoptera that threatens corn crops, one of the most important crops in the country. This research includes fruits available in the local market and classified according to international morphological markers. Proximal composition of seed meal samples was determined using the Association of Official Analytical Chemists (AOAC) methodologies, obtaining as a result: 4.29% moisture, 95.71% dry matter, 1.34% ash, 31.14% ethereal extract, 2.59% nitrogen, and 16.19% protein. To determine the insecticidal potential a bioassay was performed (48 hours) where the effect of complete seed and endosperm extracts obtained by Soxhlet extraction method were evaluated on the third larval stage of cogollero worm specimens reared in the laboratory from its hatching. The extracts were applied to the food (corn leaves) at five concentrations between 0.70 – 25.00%. The lethal concentration 50 (LC50) was 2.13% for endosperm extract and 1.85% for complete seed extract.
INTRODUCTION
Nicaragua is an eminently agricultural country with approximately 1,504,000 ha of arable soils (FAO, 2019) which are intended for the cultivation of different areas, including cereals that constitute a fundamental link for the population. Corn is probably the most important cereal in the country since it is an essential part of our diet and is used for the preparation of concentrates or animal feed (Bornemann et al., 2012).
National corn production is affected by various pests, the main one being the cogollero worm (Spodoptera frugiperda JE Smith), a lepidoptera insect that in some cases can cause the total loss of the plantation, when it occurs as an army worm (Jaramillo D., Á., Jaramillo G., O., Bustillo P., A., & Gómez L., H., 1989). Similarly, this pest is considered persistent because once the food source is depleted, the worm moves to new sources where it feeds until its development is complete.
Usually this pest is controlled with synthetic products such as the Counter 10 G nematicide (terbufos), the organophosphorus insecticide Lorsban 4 EC and pyrethroids such as Cypermethrin combined with Diazinon (Espinoza et al., 1999; INTA, 2010), which harm the health of producers and their families, in addition to contaminating the environment and the food in which they are applied. In Nicaragua, the incidence of pesticide associated poisonings is a public health priority given the high incidence and mortality that it represents (Pardo Abdala, L.M., Pérez Rodríguez, S., & Gámez Bacallao, A., 2017). Additionally, a collateral damage of these products is the affectation to species or organisms that are not harmful or beneficial to the native ecosystem of the crop site.
In this context and with the objective of reducing the negative impact of traditional chemical treatment, there is an international trend in seeking more ecological and sustainable alternatives that includes the use of plant extracts for pest control. Within this group of plants with insecticidal potential we find soursop (Annona muricata L.) whose properties are associated with acetogenins, secondary metabolites responsible for their characteristics and present throughout the tree (Arroyo et al., 2005; Bobadilla et al., 2005).
In Nicaragua, soursop is popularly known for the taste of its pulp and its consumption as fresh fruit. The leaves and seeds are used in traditional medicine for their antitumor, parasiticidal and antidiarrheal capacity (Santos Pimenta et al., 2003). At an international level its economic importance has grown to project itself in the perfumery, pharmacology and agricultural industry.
Although there are studies at international level, to expand the applications of the national soursop as a raw material, its morphological characterization and the determination of its proximal composition is imperative because the edaphoclimatic conditions directly influence the quantity and quality of the cultivars as well as its nutritional content (Peckham G., 1979). This research aims to contribute to this end by establishing toxicity tests (bioassays) that assess the soursop’s insecticidal potential on the cogollero worm and determining the proximal composition of the seeds to promote their use as a raw material.
MATERIALS AND METHODS
Sample collection
Thirty fruits were collected in the trade of the departments of Masaya and Carazo during the months of July and August taking into account their state of physiological maturity. The samples were transported to the Biotechnology Laboratory of the Universidad Nacional Autónoma de Nicaragua, Managua (UNAN-Managua) where they were disinfected and processed under controlled conditions to prevent degradation. The seeds were extracted from the fruit, washed with drinking water and distilled water, dried at room temperature for 7 days and in a convection oven at 40°C for 24 hours.
A portion of the dried seeds were separated from the pericarp in order to release the endosperm to analyze the concentration of the insecticidal components of both the endosperm and the whole seed. Finally, both samples were macerated and homogenized in a Retsch mill with integrated sieve (0.5 mm pore size). The flour obtained was stored in amber airtight containers at 4°C.
Morphological characterization of the samples
Soursop fruits, as well as the extracted seeds, were characterized according to the morphological markers internationally established by Bioversity International and CHERLA (2008) adapted for the species. Some of these parameters were: length, diameter, weight of the fruit, weight of seeds, as well as anatomical characteristics of the shell (exocarp), pulp (mesocarp) and seeds.
Determination of the proximal content of the seed
The chemical composition was obtained using official working methodologies established by the AOAC. The determination of humidity, ash, proteins and ethereal extract were performed according to: AOAC 925.1, AOAC 923.03, AOAC 2001.11 and AOAC 920.39, respectively. All analyzes were performed in triplicate with negative controls (white) and internal quality controls for the verification of the results. The data obtained were evaluated to determine the averages and standard deviation of the triplicate samples.
Quality control of proximal parameters
Analytical procedure controls were included such as: laboratory replicas, laboratory targets, enriched and aggregates. The reproducibility of the results was evaluated by means of replicas of the sample to demonstrate its degree of homogeneity and representativeness. The accuracy of the results obtained is reflected through the relative standard deviation (RSD). Accuracy was evaluated from the recovery percentages obtained in the white and enriched soursop seed samples. These are used to demonstrate the reliability of the method and are considered acceptable when the recovery of 90% of the analytes of interest is between 90.00 and 105.00% of the concentration added or enriched.
Obtaining and maintaining the larval brood of Spodoptera frugiperda J. E. Smith
To guarantee the identity and health of the specimens included in the bioassay, a breeding stand was made with egg masses of the cogollero worm (10) obtained from the Entomology Laboratory of the National Autonomous University of Nicaragua, León (UNAN-León). Egg masses were kept in Magenta™ polycarbonate containers until the first larval stage. They were exposed to photoperiods of 12 light hours and 12 dark hours, with 60% relative humidity and 26 ± 3°C ambient temperature. Upon reaching the second larval stage, they were individualized in test tubes where they were maintained until the third larval stage, when they were moved to glass jars. The larvae were fed every 24 hours with rectangles of previously disinfected corn foliar tissue.
Extractions by Soxhlet method
15g of soursop seed and endosperm flour were weighed in duplicate and placed in a hemicellulose thimble inside the Soxhlet equipment where 100ml of the solvent (96% ethanol) was added under reflux for 16 hours. The extracts were stored in airtight and dark containers at room temperature for one week and subsequently in refrigeration (4°C) until use.
Preparation of treatments
For the application of the extracts (endosperm and complete seed) five concentrations or treatments were defined: 0.70%, 3.00%, 7.00%, 15.00%, 25.00%. The solvent used for its preparation was distilled and degassed water at 50°C, maintaining constant stirring during dilution. The treatments were prepared the same day of the application to avoid precipitation or biphasic behavior and were stored in amber containers to avoid their degradation by light.
Performing the bioassay
Based on the entomological test guidelines described by Roberson, et al. (2007) and Leatemia & Isman (2004) adapted to laboratory conditions. The cogollero worm larvae were fed with three rectangles of corn foliar tissue previously submerged in 40ml of the different treatments to the point of runoff. The food was changed every 24 hours, however only the first portion of food was treated to avoid doubling the dose. Mortality was determined based on the response of the larvae to the stimulus with a brush and mortality counts were performed every 8 hours. Evidence of feeding was also verified every 24 hours, prior to the change of food. The insecticidal potential of each matrix (endosperm and complete seed) was evaluated in the five treatments mentioned above (0.70%, 3.00%, 7.00%, 15.00%, 25.00%). Each treatment was evaluated in sets of 10 individualized specimens in bottles and in triplicate (30 larvae for each treatment), totaling 150 individuals (300 larvae for both matrices).
Likewise, the following were included: negative controls formed by larvae fed with untreated tissue to corroborate that there were no deaths associated with the maintenance conditions of the species; ethanol control to underestimate the insecticidal activity of ethanol that was used as a solvent in the extractions; finally a control without food to verify the survival of the larvae without plant material. The distribution of the larvae in the controls was the same applied in the treatments (10 individualized larvae in triplicate for each control), totaling 90 specimens. 390 larvae were used for the entire bioassay. It should be noted that in order to promote feeding, the specimens under study were not fed within 24 hours prior to the bioassay.
Analysis of data
The morphological characterization of the seed and fruit were averaged (quantitative data) and its frequency was determined (qualitative data). The results of the replicas of the proximal analysis were averaged and compared with international references from Congo (Kimbonguila et al., 2010) and Venezuela (Vit P., Santiago B. and Pérez Pérez E. M., 2014).
On the other hand, for the analysis of the insecticidal potential, the LC50 was determined based on the mortalities of each extract calculated using the average and by the Probit regression analysis. The effectiveness was stipulated based on the yield obtained by each of the extracts based on the mortality of the larvae, performing an analysis of variance (ANOVA) to find the difference between the mortality rates of each. It was considered as the most effective concentration, the one that in less concentration eliminates 50% of the individuals or as close as possible to half. The assumptions of normality and homogeneity of variance were made using the InfoStat program (Balzarini et al., 2008; Di Rienzo et al., 2011). Fisher comparisons were subsequently made to identify the existence or absence of significant differences between the groups.
RESULTS AND DISCUSSION
Morphological classification
The highest frequency percentages found correspond to fruits with an elongated cordiform shape (53.30%) (Figure 1), exocarp of small bumps (50.00%) (Figure 2) and dark green color (36.70%) (Figure 3).
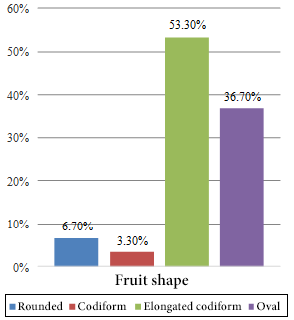
Figure 1. Frequencies of fruit shape.
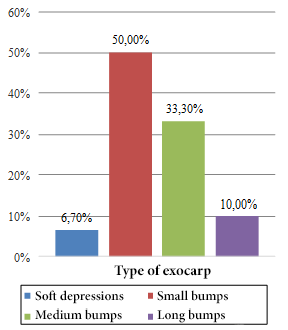
Figure 2. Frequencies of the fruit exocarp type.
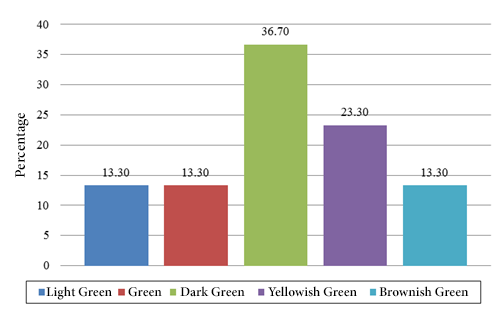
Figure 3. Exocarp color frequencies.
In relation to the seeds the predominant color was brown (80.00%) (Figure 4) and in the case of the pulp the most predominant color was white (53.30%) (Figure 5) similar to the value obtained to the sweet taste (53.30%) (Figure 6).
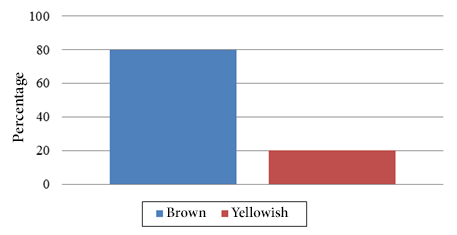
Figure 4. Seed color frequency.
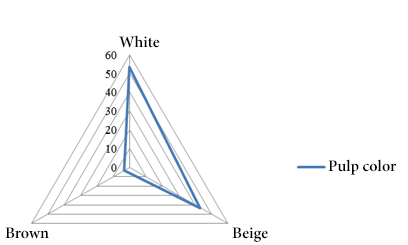
Figure 5. Pulp color frequencies.
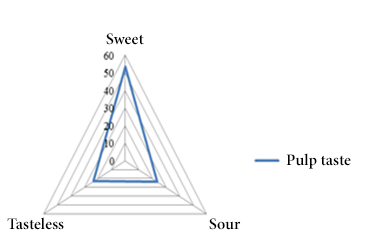
Figure 6. Pulp taste frequencies.
Proximal composition
The content of ethereal extract (31.14 ± 0.56%) was the proximal parameter with greater predominance within the dry matter of the soursop seed. In lower contents and in decreasing order were found the protein content (16.19 ± 0.16%), nitrogen (2.59 ± 0.16%) and ashes (1.34 ± 0.03%) (Figure 7). The humidity and dry matter determined were: 95.71 ± 0.02% and 4.29 ± 0.02%, respectively (Figure 8).
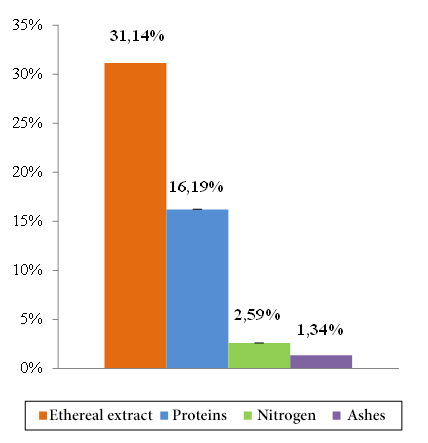
Figure 7. Proximal composition and relative standard deviation of the soursop seed.
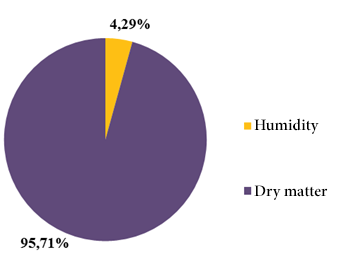
Figure 8: Moisture and dry matter content of soursop seed.
When comparing these results with the values reported in the literature, it can be observed that the soursop seeds of Masaya and Carazo have the highest dry matter values and the lowest humidity values with respect to seed meal in Mpissa, district of southern Congo (Kimbonguila et al., 2010) and Mérida, Venezuela (Vit et al., 2014). The national seed also reports higher total fat content (ether extract) and proteins than those obtained by Vit et al. (2014) However, the seeds of Merida, Venezuela have a higher percentage as far as ashes are concerned (1.44%). On the other hand when comparing the results reported by Kimbonguila et al. (2010) higher values of ash and ethereal extract (9.70%, and 40.00%, respectively) are evidenced, this considerably higher value of the lipid fraction arouses interest in its use in relation to nutritional quality of food, although it reports a value lower in protein (8.50%) compared to that obtained during the analyzes (16.19%) (Figure 9).
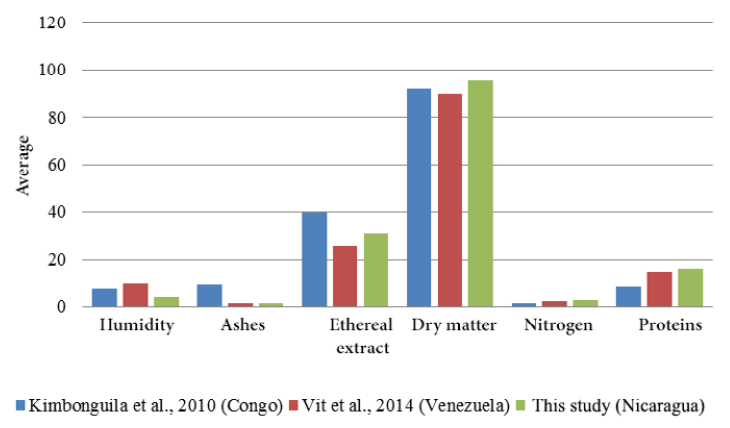
Figure 9. Comparison of the composition of soursop seeds in different countries.
The variation in the proximal contents, despite being the same species, may be due to differences in the climatic and soil conditions of each country, providing more or less nutrients to the plant, also influences the variety of soursop that is used for analysis (acid, sub-acid or sweet).
Quality control of proximal parameters
Table 1 summarizes the results of the quality controls performed for each of the analyzes, which were compared with the results interpretation guide established by the AOAC, determining that they are all within the permissible ranges (% RSD ≤ 1.90 and% recovery: 90.00-105.00%).
Table 1. Quality controls of the proximal parameters in soursop seed meal.
|
Parameters |
Quality controls |
% RSD |
% Recovery |
|
Humidity |
Sample in triplicate |
0.27 |
- |
|
Enriched sample (added) |
1.62 |
100.55 |
|
|
Ashes |
Sample in triplicate |
1.80 |
- |
|
Ethereal extract |
Sample in triplicate |
1.80 |
- |
|
Enriched sample |
0.03 |
96.96 |
|
|
Enriched white |
1.03 |
98.09 |
|
|
Proteins |
Sample in triplicate |
0.27 |
- |
|
Enriched white |
- |
94.07 |
Average lethal concentration (LC50) of extracts
The LC50 of the endosperm ethanolic extract of the soursop seed samples was 2.13% (ƿ = 0.0001) (Figure 10). This result shows that the LC50 is between the lowest concentrations or treatments used for the bioassay (0.70% and 3.00%) that caused a lethal effect on 3.33% and 86.66%, respectively. From the concentration of 7.00% to 25.00%, 100.00% mortality was recorded for this extract.
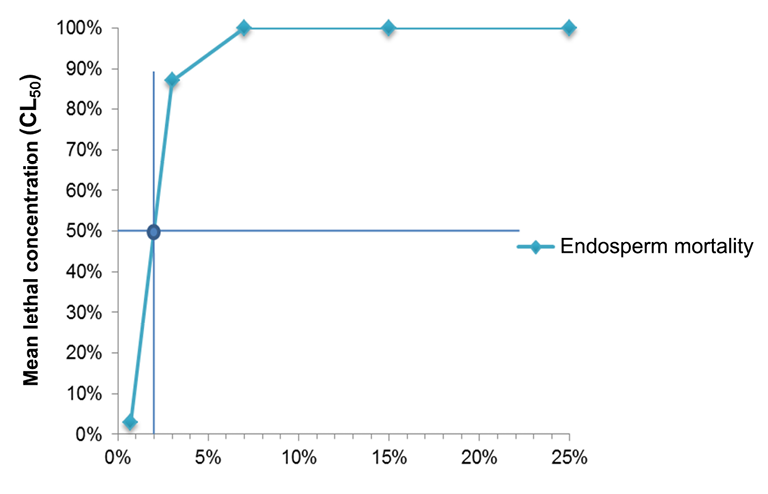
Figure 10. Mean lethal concentration (LC50) of the seed endosperm extract of Annona muricata L. prepared by the Soxhlet method.
For the ethanolic extracts obtained from the complete seed of Annona muricata L. the LC50 of 1.85% (ƿ = 0.0001) was calculated (Figure 11). This result of the Probit analysis indicates that the LC50 is among the lowest treatments used for the bioassay (0.70% - 3.00%) that caused death to 3.33% and 96.66% of the population, respectively. From the concentration of 7.00% to 25.00%, 100.00% mortality was recorded for the extract.
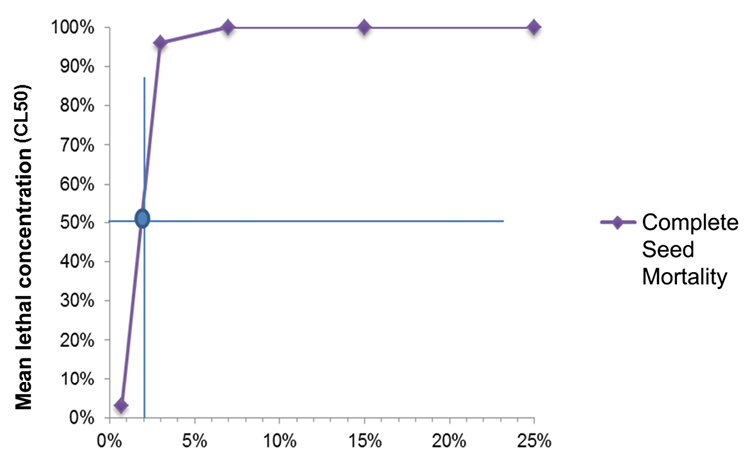
Figure 11. Mean lethal concentration (LC50) of the complete seed extract of Annona muricata L. prepared by the Soxhlet method.
There is an evident similarity in the results obtained by both extracts: the LC50 is among the lowest bioassay treatments and the higher doses reach 100.00% lethality over the larval population. The difference between the values of both extracts (endosperm and complete seed) provides the guidelines to suggest the complete seed as the extract that requires less concentration to cause a lethal effect on half of the insect population in a time of 48 hours after the application.
When evaluating the results obtained with international references, the coincidence with what was established by Ángel-Ríos MD, Pérez-Salgado J. and Morales De Jesús F. (2015) was identified for the effect of the seed extract of A. muricata L. obtained by Soxhlet on the cogollero worm and analyzed at a concentration of 2.00%. In this study conducted in Mexico, it was defined that under the conditions described above, the extracts caused 87.97% of mortality on the larvae, similar to the mortality obtained in the treatment of 3.00% in the present investigation (96.66%).
Acute toxicity was analyzed based on data collected during the bioassay (48 hours), however the median lethal concentration was not a constant over time, which is why several counting times (every 8 hours) were established and The LC50 was calculated according to the ecotoxicological principles of Walker, CH, Sibly, RM, Hopkin, SP, & Peakall, DB (2012). The curve in Figure 12 shows how acute toxicity decreases as the exposure time increases until reaching the threshold concentration near 48 hours where the curve begins to parallel with the asymptote X, however between the last two counting times (40-48 hours) a variation of the LC50 is distinguished from 2.20% to 2.13% respectively, indicating that the threshold concentration is not definitive and that mortality may vary with the increase in the observation time (more than 48 hours), up to that acute toxicity ceases to vary over time becoming a straight line.
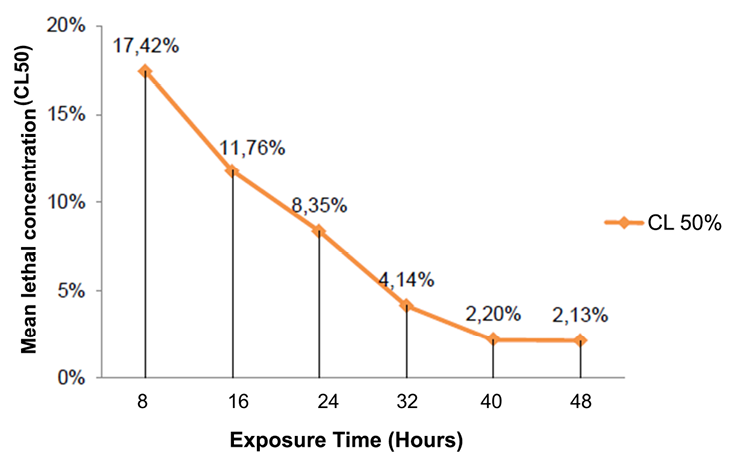
Figure 12. Mean lethal concentration (LC50) in the exposure time of the seed endosperm extract of Annona muricata L. on the larvae of Spodoptera frugiperda.
In the curve of the lethal median concentration through the exposure times during the 48 hours for the complete seed extract (Figure 13) it was appreciated how the curve decreases until 40 hours and, opposite to what happened with the endosperm extract , a totally linear behavior is acquired with respect to the X axis reaching its definitive threshold concentration, that is to say that no changes in mortality will be experienced even if the exposure time is increased, perfectly complying with the ecotoxicological principles of Walker et al. (2012). When the threshold is reached the whole experimental system is stabilized, therefore the concentration of toxins in the larval organism could not increase or decrease regardless of whether they ate food or not, during and after 48 hours, thus the subjects who achieved surviving the bioassay, they survived over time by coping with toxins in their metabolisms.
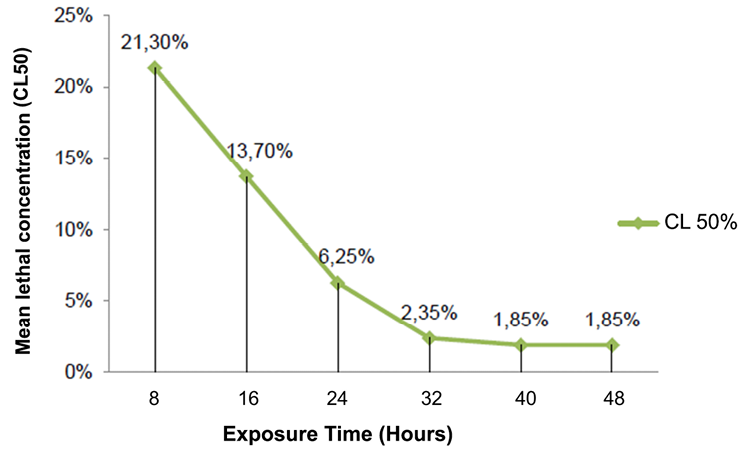
Figure 13. Mean lethal concentration (LC50) in the exposure time of the complete seed extract of Annona muricata L. on the larvae of Spodoptera frugiperda.
The time interval with the highest mortality was 8 hours after the application of the extracts with a total of 110 larvae (73.33%) for the endosperm extract and 113 larvae (75.33%) for complete seed.
The determination of the LC50 indicates that both the endosperm extract and complete seed have the toxicity necessary to cause the death of 50% of the population of larvae at low doses and even the entire population in concentrations greater than 3.00%, however, in order to verify its activity in situ, a field validation must be carried out with a standardized concentration, mainly of the complete seed extract, which proved to be the most efficient because it required the lowest concentration to reach LC50.
Anti-food effect
During the bioassay the food intake by the larvae was recorded and it was observed that there is an inverse behavior between the concentration of the extract and the evidence of ingestion of the foliar tissue of corn used as food: when the concentration or treatment was increased it was recorded higher mortality with less evidence of feeding. This observation allowed to infer that the extracts have a repellent or anti-food effect on the larvae and combined with their contact toxicity caused the death of the larvae, coinciding with that reported by Leatemia and Isman (2004). In this way it can be seen that the extracts act by ingestion, by contact or repellency, raising the potential of A. muricata L. as an alternative for the control of the cogollero worm.
ANOVA of the concentrations applied to the larvae
The analysis of variance and the Fisher comparison showed that the concentrations of the two extracts (endosperm and whole seed) between 3.00 and 25.00% did not show significant differences in the mortality average of S. frugiperda larvae, but there is a difference between them and the concentration of 0.70% that was significantly lower (ƿ = 0.0001, F = 35.85) (Figure 14).
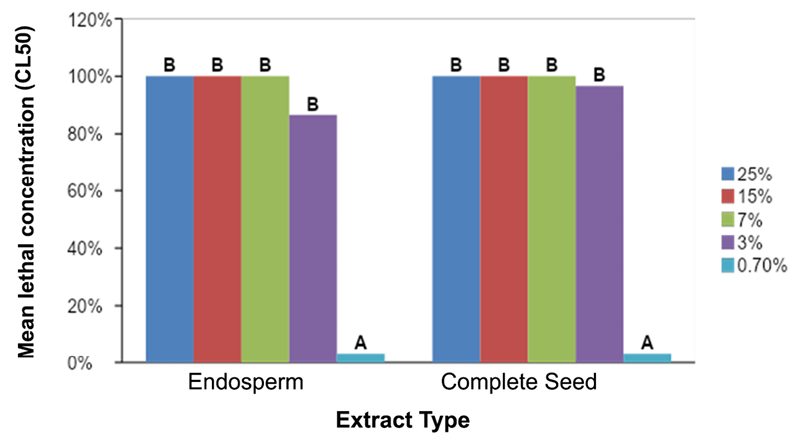
Figure 14. Comparison of the mean mortalities reported for the different treatments prepared from endosperm extracts and complete seed of A. muricata L. on the larvae of S. frugiperda. The treatments identified with the same letter do not differ statistically.
The similarity between the mortalities recorded for both extracts may be due to the fact that the acetogenins, which cause the insecticidal effect, are distributed in a similar way in both matrices (complete seed and endosperm) because it is in these portions where the lipid portion is concentrated of the soursop seed. It should be noted that during the preparation of the treatments, both matrices showed the same biphasic behavior at high concentrations (15.00 and 25.00%).
CONCLUSIONS
The morphological characterization of the analyzed samples reflected as predominant characteristics the elongated shape (53.30%), exocarp with small protuberances (50.00%) and dark green color (36.70%). Similarly, the seeds contained in the fruit are predominantly brown (80.00%) and the pulp has a white color with a sweet taste (53.33%).
The proximal analysis of the seed reflected 1.34% of ashes surpassed by reported values in samples of Mpissa, Congo, 4.29% of humidity and 95.71% of dry matter, 31.14% of ethereal extract, 2.59% of nitrogen and 16.19% of protein, the latter being similar to the published international references of Mpissa, Congo and Mérida, Venezuela.
The LC50 for the endosperm extract was 2.13% and for the complete seed extract it was 1.85%, identifying the latter as the most efficient alternative for the control of the cogollero worm because it requires less concentration to cause the mortality of 50% of the population. Similarly, during the bioassay, the anti-food effect of the extracts was evidenced with an inverse tendency to increase the concentrations.
ACKNOWLEDGEMENTS
This study was carried out within the framework of the Interinstitutional Agreement established with the Nicaraguan Institute of Agricultural Technology (NIAT) with support from the funding received by the Fund for Research Projects (FRP) of UNAN-Managua. Special thanks to Dr. Katia Montenegro Rayo for the advice given in the review of the results obtained.
REFERENCES
Ángel-Ríos M.D., Pérez-Salgado J. y Morales De Jesús F. (2015). Toxicidad de extractos vegetales y hongos entomopatogenos en el gusano cogollero Spodoptera frugiperda J.E.Smith (Lepidoptera: noctuidae), del maíz en el estado de Guerrero. Entomología Mexicana Vol. 2: 260-265 (2015).
Arroyo A, J., Prashad G, M., Vásquez B., Y., Li P, E., & Tomás C, G. (2005). Actividad citotóxica in vitro de la mezcla de Annona muricata y Krameria lappacea sobre células cancerosas de glándula mamaria, pulmón y sistema nervioso central. Revista Peruana de Medicina Experimental y Salud Publica, 22(4), 247-253.
Balzarini, M. G., Gonzalez, L., Tablada, M., Casanoves, F., Di Rienzo, J. A., & Robledo, C. W. (2008). Manual del Usuario. Córdobas, Argentina: Editorial Brujas.
Bobadilla , M., Zavala, F., Sisniegas, M., Zavaleta, G., Mostacero, J., & Taramona, L. (2005). Evaluación larvicida de suspensiones acuosas de Annona muricata Linnaeus «guanábana» sobre Aedes aegypti Linnaeus (Diptera, Culicidae). Revista peruana de Biología, 12(1), 145-152.
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Tablada, Y. C. (2011). InfoStat versión 2011. 8, 195-199. Argentina. Retrieved from http://www.infostat.com.ar
Espinoza, S. A., Urbina, A. R., Obando, S. R., & Vanegas, J. R. (1999). Cultivo del maíz. Managua, Nicaragua: Instituto Nicaragüense de Tecnología Agropecuaria. Retrieved from http://agris.fao.org/agris-search/search.do?recordID=NI2006002701
FAO. 2019. Datos sobre la tierra en http://www.fao.org/faostat/en/#data/RL, accesado el 24 de marzo del 2019.
INTA. (2010). Cultivo del maiz. Guía Tecnológica 3, Instituto Nicaragüense de Tecnología Agropecuaria, Managua.
Jaramillo D., Á., Jaramillo G., O., Bustillo P., A., & Gómez L., H. (1989). Efecto del Gusano Cogollero Spodopfera frugiderpa (J. E. Smith) sobre el Rendimiento del Maíz. Revista Facultad Nacional de Agronomía Medellín, 42(1), 25-33.
Kimbonguila A., Nzikou J. M., Matos L., Loumouamou B., Ndangui C. B., Pambou-Tobi N., et al.. (2010). Proximate Composition and Physicochemical Properties on the Seeds and Oil of Annona muricata grown in Congo-Brazzaville. Research Journal of Enviromental and Earth Sciences, 2(1), 13-18.
Leatemia, J. A., & Isman, M. B. (2004). Insecticidal Activity of Crude Seed Extracts of Annona spp., Lansium domesticum and Sandoricum koetjape against Lepidopteran Larvae. Phytoparasitica, 32(1), 30-37.
Onimawo I. A. (2002). Proximate composition and selected physicochemical properties of the seed, pulp and oil of sour sop (Annona muricata). Plant Foods for Human Nutrition, 57(2), 165-171.
Pardo Abdala, Lissett María, Pérez Rodríguez, Sonia, & Gámez Bacallao, Arianna. (2017). Reportes al Centro Nacional de Toxicología de mujeres en edad fértil expuestas a plaguicidas. Revista Cubana de Medicina Militar, Volumen 46, número 1, páginas 10-18.
Peckham, G. (1979). Foundations of Food Preparation. New York, Estados Unidos : MCMILLAN, 1979. Ref 641.5 P368 1979.
Roberson, J. L., Savin, N. E., Russell, R. M., & Preisler, H. K. (2007).Bioassays with Arthropods (Segunda ed.). (T. a. Group, Ed.) Estados Unidos: CRC Press.
Santos Pimenta, L.P., Pinto G.B., Takahashi J.A., Silva L.G.F. e, Boaventura M.A.D. (2003). Biological screening of Annonaceous Brazilian Medicinal Plants using Artemia salina (Brine Shrimp Test), Phytomedicine, Volume 10, Issues 2–3, 2003, Pages 209-212, ISSN 0944-7113, https://doi.org/10.1078/094471103321659960.
Vit P., Santiago B. y Pérez Pérez E. M. (2014). Composición química y actividad antioxidante de pulpa, hoja y semilla de guanábana (Annona muricata L.). Interciencia, 39(5), 350-353.
Walker, C. H., Sibly, R. M., Hopkin, S. P., & Peakall, D. B. (2012). Principles of ecotoxicology (4th Edition). Florida, Estados Unidos: Taylor & Francis Group. ISBN 9781439862667.