ISSN 2410-5708 / e-ISSN 2313-7215
Year 9 | No. 25 | p. 195 - 211 | June - September 2020
© Copyright (2020). National Autonomous University of Nicaragua, Managua.
This document is under a Creative Commons
Attribution-NonCommercial-NoDerivs 4.0 International licence.
Lead removal in aqueous solution using polyacrylamide-based cryogels as adsorbent: Balance study in batch mode
https://doi.org/10.5377/torreon.v9i25.9855
Submitted on February 3, 2020 / Accepted on April 19, 2020
B.A.Sc. Martha Jarquín Pascua
Laboratory analysis specialist
Biotechnology Laboratory, UNAN-Managua
Ph.D. Martha Lacayo Romero
Professor researcher
Biotechnology Laboratory, UNAN-Managua
Keywords: MPAAG-TBA cryogels, Lead (Pb2+), removal, adsorption capacity, equilibrium isotherms
Abstract
The objective of this research was to remove lead (Pb2+) ions from aqueous solutions using macroporos polyacrylamine gel (MPAAG) called cryogels as an adsorbent material. The MPAAG, were prepared by a free radical co-polymerization reaction of acrylamide (AAm) and allyl glycidyl ether (AGE) and N, N-methylene-bis (acrylamide) (MBAAm) bonded to epoxy functional groups (epoxy-pAAm) at a concentration of 7,5%, the reaction was carried out at -12 oC for 1 hour. After the preparation process, MPAAG was evaluated by binding the tris (2aminoethyl) amine (TREN) ligand groups followed by bromoacetic acid (BA). The presence of the amide and carboxyl group in the structure of the cryogels allowed the removal of lead to apply in aqueous solutions. The ability to remove lead on the MPAAG-TBA has been determined at different pH, metal concentrations and contact times. The technique used for the determination of lead in the aqueous solution was the Atomic Absorption Spectrophotometer (AAS). The maximum adsorption capacity of lead on the MPAAG-TBA was 10,78 mg/L at pH5 in 30 minutes. Adsorption equilibrium was performed at concentrations of 20,72; 41,44; 82,88 and 165,75 mg/L respectively of lead ions on the MPAAG-TBA results obtained were adjusted with the Langmuir and Freundlich model. The maximum adsorption capacity was determined by the Lagmuir model, the adsorption intensity (RL) values were lower than one, indicating that the adsorption of lead on the MPAAG-TBA is favorable; however it did not fit the model in the first two concentrations. The regression coefficient (R2) was used the Freundlich model and the intensity values (n) were found in the range of 1 to 10 considering the process of lead removal on the MPAAG-TBA was favorable. The results indicate that MPAAG-TBA criogels could be used as adsorbent material to remove ions Pb2+ present in aqueous solution at low concentration.
Introduction
Currently, there is great concern worldwide, due to the considerable increase in the rates of contamination of industrial effluents by heavy metals and, in turn, they are considered potentially dangerous in living organisms due to their chemical transformations and bioaccumulation (Tejada -Tovar, Villabona-Ortiz, & Garcés-Jaraba, 2015). Lead (Pb2+) has been recognized as one of the most dangerous heavy metals, the main sources of contamination come from mining and smelting activities, the combustion of leaded gasoline, the application of sewage sludge to the soil, the disposal battery and lead-containing products (Huang et al., 2006). According to the World Health Organization (WHO), it established that the maximum concentration of heavy metal ions in water should be in a range of 0.01-1 ppm, however, ion concentrations are currently reported of heavy metals up to 450 ppm in the effluents (Tejada-Tovar et al., 2015).
Many processes and technologies have been developed and applied to remove heavy metal ions from water, including chemical precipitation, extraction with ion exchange solvents, electrode deposits, and adsorption of activated carbon are well known but expensive. The use of adsorbents is one of the most effective methods from both an economic and technological point of view (Halah, Halah, & López-carrasquero, 2018); However, many researchers from other countries have shown their interest in this material and have been introduced as a new matrix for applications in various bioseparation processes (Carvalho et al., 2014), such as chromatography of biological nanoparticles and microparticles (Plieva et al., 2005), as well as the separation, purification, release of drugs, biological, pharmaceutical, medical and tissue engineering environmental contaminants (Sofia & Duarte, 2012; Zdravkovic, Nikolic, Ilic-Stojanovic, & Nikolic, 2017).
In Nicaragua the study of cryogels as adsorbent material is currently being carried out in laboratory scale environmental applications. Poly (acrylamide) -based cryogels (pAAm) have been prepared using the cryogelation technique (Ertürk & Mattiasson, 2014), polymerization reactions at low temperatures occur when most of the solvent (water) freezes while the reagents dissolved are concentrated in small, non-frozen regions, called “liquid microphases.” Gel formation in liquid microphase and frozen solvent crystals work as porogens (Plieva et al., 2005), these characteristics make them attractive for the direct capture of biomacromolecules (Carvalho et al., 2014). The cryogel water retention, the porosity of the network and the elastic properties allow them applications as adsorbents for various metal ions present in aqueous solution. During the adsorption process, various authors mentioned the formation of cryogel metal complexes through the addition of functional groups (eg, –COOH, –SO32–, –C = O (NH2), –SH, –COO–, -NH2 and –OH) (Halah, Halah, & López-carrasquero, 2018). The binding of suitable functional groups to the cryogel matrix is, furthermore, a primary key to a wide range of application areas, for example, capturing pharmaceuticals, protein binding or cell immobilization (Onnby, Giorgi, Plieva, & Mattiasson, 2010).
The search for alternatives to reduce water contamination by lead through discharges of effluents from various industrial and agricultural activities, this study aimed to remove the lead ions present in the aqueous solutions especially when it is found at low concentrations through the use of an adsorbent material called cryogel that are linked to functional groups such as primary and carboxylic amines (MPAAG-TBA).
Materials and Methods
Materials and reactives
Acrylamide (AAm, > 99.9% pure, electrophoresis grade), allyl glyceryl ether (AGE, 99%), N, N'-methylene-bis (acrylamine) (MBAAm) and ammonium persulfate (APS), bromoacetic acid, N, N, N', N'-tetramethyl-ethylenediamine (TEMED), tris (2-aminoethyl) amine (TREN), 2,4,6-nitrobenzenesulfamic acid (TNBS) were from Sigma-Aldrich, lead acetate trihydrate (CH3COO)2.3H2O)), reactive grade hydrochloric acid (HCl) and sodium hydroxide (NaOH), nitric acid (HNO3), sodium carbonate (Na 2CO3) and sodium bicarbonate (NaHCO3), 1000 mg/l-1 standard solution -1 Pb certified.
Preparation of the lead solution
In the experimental batch mode, aqueous solution of lead ions was used and they were prepared at different concentrations (20.72; 41.44; 82.88 and 165.76 mg/L) from the Pb salt (CH3COO)2.3H2O. For the determination of the concentration of lead in the aqueous solution, the Atomic Absorption Spectrophotometer (AAS) equipment was used by preparing a working range using a certified standard solution of 1000 mg/L at a wavelength of 283.3 nm.
Method for the polymerization of the epoxy pAAm
The pAAm-cryogels with epoxy functional groups (epoxy pAAm) have been produced according to the study carried out by Onnby, et.al., 2010 and Plieva et.al., 2005. The total monomer content was added in an erlemeyer 7, 6 g of acrylamide; 3.2 g of MBAAm and 1.0 ml of AGE were dissolved in 170 ml of deionized water to obtain a final concentration of 7.5 w/v%. Then it was degassed for 30 minutes, 190 µl TEMED was added. The content was placed in an ice bath for 30 minutes. Subsequently 152.6 mg of APS was added, the mixture was quickly stirred, then they were poured into the glass columns (15 ml, ID 9 mm) containing the carriers (molds) and they were placed in a thermostatic bath at -12 °C for 1 h, then they were transferred to a freezer at -12 °C for one hour. The tubes were left at room temperature for 30 minutes and were cut according to the diameter of the carrier and placed in deionized water. Stirring washes at 100 rpm for 1 hour for three consecutive times and were then stored at 4 °C.
Addition of ligand groups (amines and carboxyl)
After the preparation of the epoxy-MPAAG, the addition of the carboxylic groups was carried out as follows: the first reaction consists of adding ligand groups that are the primary amines, the carbonate buffer was prepared (NaHCO3: Na2CO3) 0.2 M at pH = 9.2-9.4 at a volume of 800 ml and 5.04 ml of tris (2aminoethyl) amine (TREN) was added, then the epoxy-MPAAG that was obtained from cryogelization was added, left stirring for 15 h at 200 rpm, then the cryogel was washed with deionized water to remove excess reagent.
To verify the binding of the TRAIN group in the epoxy-MPAAG, it was necessary to take the gel at random, it was placed in a vial containing 1 ml of sodium carbonate pH = 8.5, 2 μl of trinitro benzene-sulfonic acid were added (TNBS). The yellow color change indicating that the ligand was correctly bound (see figure 1).
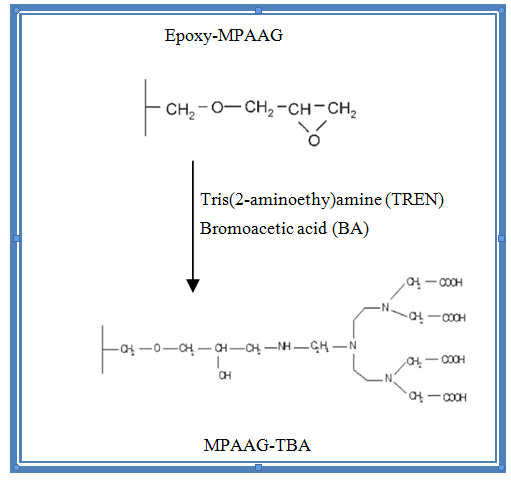
Figure 1. Epoxy-MPAAG reacts with the TREN and BA binders to obtain the MPAAG-TBA adsorbent material. Figure taken from (Onnby et al., 2010).
The second reaction was carried out by adding a carboxyl group from bromoacetic acid (BA) with a 4:1 ratio (bromoacetic acid: MPAAG) at pH 8.7, which is bound by the primary amines of the first reaction. The reaction was carried out for 20 h at 300 rpm. The pH was adjusted with concentrated NaOH, the solution must remain above 8.5. The excess reagent was removed by rinsing the MPAAG-TBA with deionized water.
Analytical methodology to determine the influence of pH and contact time on the MPAAG-TBA
The method used for this study was as described in its publication Önnby, et. al., 2010, in batch mode using 300 ml of aqueous Pb (CH3COO)2.3H2O solution at a concentration of 207 mg/L to determine the pH efficiency over the MPAAG-TBA over time. The pH was experimentally evaluated in the range of 2, 3, 4, 5 and 6, the adjustment was made with 0.1 M NaOH or 0.1 M HCl, 25 carriers containing adsorbent gel equivalent to 0.56 g were used (dry weight). 10 ml aliquots were taken between 5 to 120 minutes at laboratory temperature (24.5 ± 2 °C) with a stirring of 200 rpm. The samples were analyzed directly on the Atomic Absorption Spectrophotometer (AAS) equipment.
Sothermal adsorption balance
The isotherms and kinetics of adsorption processes provide information that determines the mechanisms and dynamics of adsorption. These mechanisms describe the amount of molecules of the adsorbate per mass unit of the adsorbent as a function of the equilibrium concentration in the solution at a constant mass and temperature (Bergmann, 2015; Dubey, Gusain, & Sharma, 2016).
There are different isotherm models to determine the experimental adsorption balance of an adsorbate in an adsorbent (Bergmann, 2015). In this study, the experimental equilibrium data of Pb2+ adsorbed on MPAAG-TBA were analyzed using the Langmuir and Freundlich isotherm models.
The content of Pb2+ ions in the aqueous phase before and after the adsorption process was determined using the Atomic Absorption Spectrophotometer (AAS). The adsorption capacity, Qe (mg / g) was calculated according to the following equation:
(1)
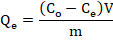
Where C0 is the initial concentration of the metal in the solution (mg/L); Ce is the concentration of the metal at equilibrium (mg/L); V is the volume of the solution (L) and m is the mass of the adsorbent (g) (Pourjavadi, Abedin-Moghanaki, & Hosseini, 2016).
Langmuir isotherm model
It is the most widely used model and is based on monolayer coverage occurring at specific homogeneous sites on the adsorbent surface that have a constant number of identical and energetically equivalent adsorption sites without any deviation to the plane of the adsorbent surface (Boparai , Joseph, & O’Carroll, 2011; Dubey et al., 2016). The non-linear form of the Langmuir isotherm is as follows:
(2)
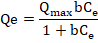
Equation 2 can be expressed linearly as described:
(3)
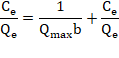
Where Qmax represents the maximum adsorption capacity of the metal (mg/g) and b is the adsorption constant (L / mg) and is related to the affinity and energy of the binding sites. The numerical values of Qmax and b are obtained from the slope and the intercept, respectively.
The essential characteristics of the Langmuir model can be expressed by means of an dimensional constant called the separation factor or equilibrium parameter (RL) (Ayawei, Ebelegi, & Wankasi, 2017; Bulut & Baysal, 2006; Foo & Hameed, 2010; Özcan, Gök , & Özcan, 2009; Sari, Tuzen, Citak, & Soylak, 2007). RL is calculated in the amplitude of the initial concentrations of the process according to the following equation:
(4)
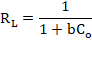
Where b is the Langmuir adsorption constant (mg / g) and Co is the initial concentration of the adsorbent (mg / L). RL values indicate the nature of adsorption and is considered the most reliable indicator for the adsorption isotherm. There are four probabilities of the value of RL, it is not favorable when RL > 1, linear when RL = 1, favorable when 0 < RL < 1 and irreversible when RL = 0.
Freundlich isotherm model
It is based on multilayer sorption on heterogeneous surfaces that support sites of variable affinity and with a uniform distribution of reversible energy and adsorption (Dubey et al., 2016). The exponential equation states that the concentration of the adsorbate at the adsorbent surface increases as the concentration of the adsorbate increases. Theoretically, using this expression, an infinite amount of adsorption will occur. Similarly, adsorption could occur through multiple layers instead of a single layer. The equation has a wide application in heterogeneous systems (Bergmann, 2015).
The nonlinear form of the equation is written as follows:
(5)
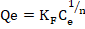
The linear form of equation 5 is shown as: (6)
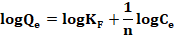
Where KF and n are the Freundlich constants related to the adsorption capacity (mg/g) and the adsorption intensity respectively. The adsorption intensity (n) is the constant of adsorption equilibrium, whose value is indicative of the heterogeneity of the adsorbent surface (Pliego-Arreaga, Regalado, Amaro-Reyes, & García-Almendárez, 2013); that is, the values indicate the degree of non-linearity between the concentration and the adsorption in the following way: the adsorption is linear if n = 1; adsorption is a chemical process if n <1, adsorption is a physical process if n>1 and if n is in the range of 1-10 it indicates that adsorption is favorable (Borhade & Kale, 2017; Desta, 2013) ; KF (mg/g (L/mg)1/n) represents the amount of metal ions adsorbed on the adsorbent for a unit of equilibrium concentration and Ce (mg/L) is the equilibrium concentration (Dubey et al., 2016).
Results and discussions
Polymerization and incorporation of the ligand groups
The preparation of MPAAG has been studied as described by Önnby, et.al., 2010. In figure 2 it is observed that the polymerization process of the MPAAG achieved at temperature -12 ºC by using the Isotemp thermostat. As mentioned in the methodology, the process of incorporation of the amine and carboxylic groups was carried out in two stages. Firstly, the addition of the ligand amine groups (TREN) was carried out, demonstrating by changing the yellow color, it also indicated that it was added correctly, being available for the next step which is the binding of the carboxylic group (BC).

Figure 2: Polymerization of MPAAG at 7.5%, -12 ºC for 2 hours and incorporation of TRAIN and BA.
The determination of the dry weight of the adsorbent gel (MPAAG-TBA) was carried out at a temperature of 60 ºC for 24 h, therefore each carrier contains 0.022 g of gel.
Influence of pH and contact time on the MPAAG-TBA in batch mode
The pH of the solution is one of the important factors that affect the adsorption behavior, due to its impact on the functional groups of the adsorbent, as well as the solubility of metal ions. Therefore, adsorption must be carried out in the optimal pH range (Pourjavadi et al., 2016). In Graph 3 (a and b) it is observed that the pH range was from 2 to 6. The results indicated that the presence of the carboxylic groups on the MPAAG-TBA demonstrates its ability to adsorb Pb2+ with increasing pH; however, at pH 6 a decrease occurred, due to the formation of precipitate in the aqueous solution, according to Qaiser, et. al, 2009, the precipitate can be attributed to the ratio of H+ ions, as a result of the exchange between Pb2+ and the proton, and also causes supersaturation of the cryogel pores, in turn deterioration of the adsorbing material. The maximum capacity (10,781 mg / g) for Pb2+ adsorption was at pH 5 where it reached equilibrium in approximately 30 minutes. PH 5 was selected as optimal for the adsorption study.
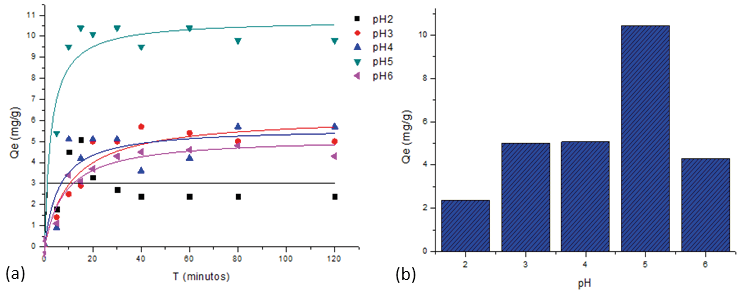
Figure 3. Effect of pH and time of contact with MPAAG-TBA on the maximum adsorption capacity of Pb2+ Langmuir, C = 207 mg/L, m = 0.56 g in 300 ml at 24.5 ºC, 200 rpm, 120 minutes. Software origin pro8 V2019 nonlinear analysis
Contact time is one of the important factors for the economic application of wastewater treatment (Abdul-Raheim, El-Saeed Shimaa, Farag, & Abdel-Raouf Manar, 2016; Pourjavadi et al., 2016). To establish the contact time between the aqueous solution containing 207 mg/L Pb2+ ions on MPAAG-TBA, the experiment was carried out in a range of 5 to 120 minutes. Figure 3 (a) shows that the maximum adsorption capacity took place during the first 30 minutes within the Pb2+ ion removal process, this was due to the availability of binding groups such as carboxylic acids, as well as the cryogel resistivity as adsorbent.
Table 1 shows other comparative studies of Pb2+ removal using different adsorbents depending on the pH and the contact time with different adsorbents.
Table 1: Comparison of the MPAAG-TBA with different adsorbents used for the removal of Pb2+.
|
Adsorbents |
Qmax (mg/g) |
pH |
Time (min) |
References |
|
Fe3O4/cyclodextrin polymer nanocomposites |
64,5 |
5,5-6 |
45 |
(Badruddoza, Shawon, Tay, Hidajat, & Uddin, 2013) |
|
PAMAM-MNC |
310,0 |
5-6 |
30 |
(Pourjavadi et al., 2016) |
|
MPS-MNPs |
70,0 |
5,5 |
60 |
(Abdul-Raheim et al., 2016) |
|
AAM PEI/AAM |
18,2 19,0 |
6,0 6,0 |
48 h 48 h |
(Alam, Muyibi, & Toramae, 2007) |
|
MPAAG-TBA |
10,781 |
5,0 |
30 |
Present studio |
Isothermal adsorption balance
Kinetic adsorption studies are important in the treatment of aqueous effluents because they provide valuable information on the mechanism of the adsorption process. (Bergmann, 2015). The statistical treatment that was used for the interpretation of results and the evaluation of the process for the determination of the equilibrium parameters and adsorption kinetics were analyzed by the linear and non-linear method according to the equation described in the Langmuir isothermal model and Freundlich, using the Microsoft Excel Program and the origin Pro8 V2019.
Langmuir isotherm model
The Lagmuir isotherm is considered an equilibrium model capable of identifying the chemical mechanisms involved in the study (Eren, 2009). Figure 4 shows the concentration range in the study of Pb2+ on the adsorption capacity of MAAP-TBA of 0.56 g of dry weight at pH5, the results obtained were from the described equations of the Langmuir isotherm model and Freundlich using nonlinear analysis.
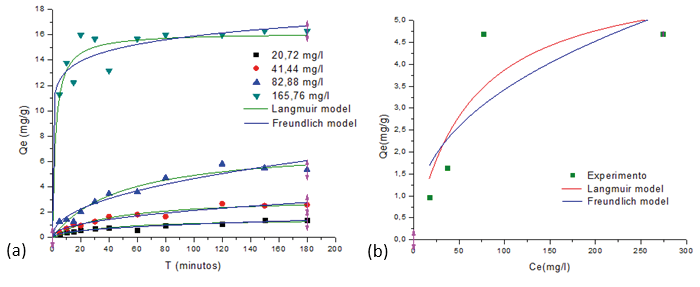
Figure 4. Adsorption equilibrium using the non-linear fit of the curve of the Langmuir and Freundlich model of isotherms of Pb2+ on the MPAAG-TBA (C = 20.72; 41.44; 82.88 and 165.76 mg/L, m = 0.56 g in 300 ml at 24.5 ºC, 200 rpm, 180 minutes Software origin pro8 V2019 nonlinear analysis
Table 2 shows that the values corresponding to the Langmuir isotherm adsorption parameters by nonlinear analysis as a function of concentration show that the maximum adsorption capacity (Qmax) was from 1,537 to 16,163 mg/g, that is, Qmax it is directly proportional with increasing concentration. The indicator of intensity or equilibrium parameter (RL) was found in an interval of 0.013 to 0.660, which indicates that it is a very favorable case (0 <RL <1). In turn, results show that when Co increases, the RL approaches to zero.
Table 2: Langmuir and Freundlich isotherm parameters for the removal of Pb2+ on the MPAAG-TBA by non-linear analysis.
|
Concentration (mg/L) |
||||
|
20,72 |
41,44 |
82,88 |
165,76 |
|
|
Langmuir |
||||
|
Qmax (mg/g) |
1,537 |
3,191 |
7,255 |
16,163 |
|
b (L/mg) |
0,025 |
0,023 |
0,021 |
0,443 |
|
RL |
0,660 |
0,507 |
0,367 |
0,013 |
|
Freundlich |
||||
|
KF (mg/g(L/mg)1/n) |
0,146 |
0,279 |
0,574 |
10,847 |
|
n |
2,338 |
2,268 |
2,203 |
12,036 |
|
1/n |
0,428 |
0,441 |
0,454 |
0,083 |
Figure 5 graphically demonstrates the applicability of the Lagmuir isotherm model using the data obtained in the study of adsorption of Pb2+ ions at different concentrations on the cryogel MPAAG-TBA, the regression coefficient (R2) was found between 0.668 to 0.992, indicating that they did not fit the Lagmuir isotherm model using linear analysis of the Origin Pro8 V2019 statistical software.
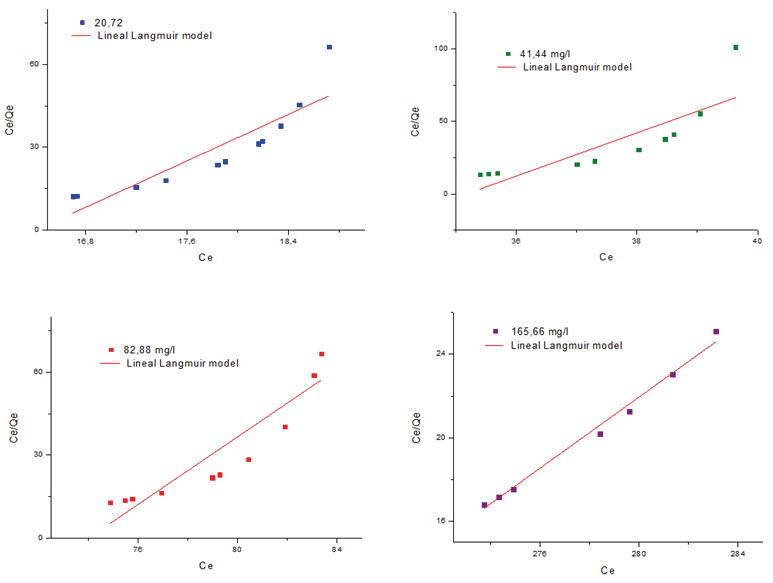
Figure 5. Langmuir isothermal model for the removal of Pb2+ on the MPAAG-TBA at different concentrations of Pb2+ ion (C = 20.72; 41.44; 82.88 and 165.76 mg/L, m = 0.56 g in 300 ml at 24.5 ºC, 200 rpm, 180 minutes Software origin pro8 V2019 linear analysis.
Table 2 shows that the Freundlich isotherm model has the same behavior as the Langmuir isotherm, since increasing Pb2+ concentrations increases KF from 0.146 to 10.847 (mg/g(L/mg)1/n). According to (Alam et al., 2007) the Langmuir (Qmax) and Freundlich (KF) constants have different meanings, but the same conclusion is reached regarding the correlation of the experimental data with the adsorption model. The basic difference between Qmax and KF is that the Langmuir isotherm assumes adsorption-free energy independent of surface coverage and monolayer formation, while the solid surface reaches saturation, the Freundlich isotherm does not predict saturation of the solid surface by adsorbate, and therefore the coverage of the surface is mathematically unlimited.
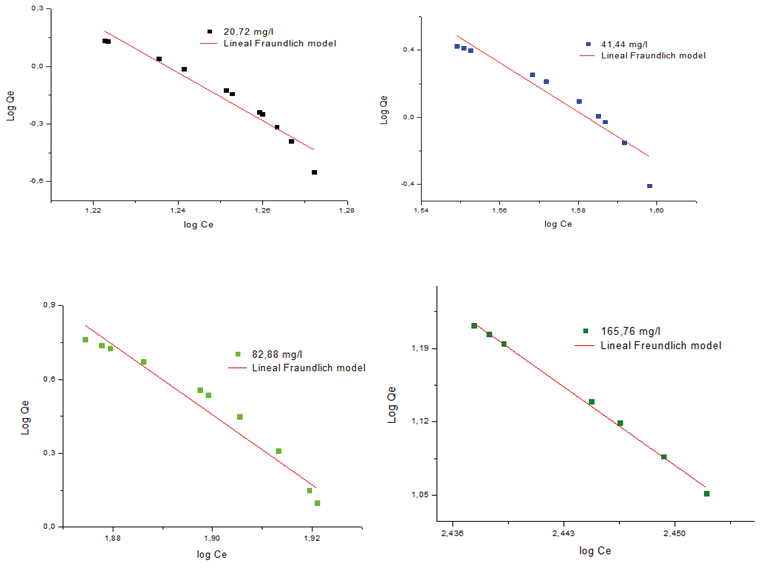
Figure 6. Freundlich isotherm model for the removal of Pb2+ on the MPAAG-TBA at different concentrations of Pb2+ ion (C = 20.72; 41.44; 82.88 and 165.76 mg/L, m = 0.56 g in 300 ml at 24.5 ºC, 200 rpm, 180 minutes Software origin pro8 V2019 linear analysis.
The Freundlich isotherm through nonlinear analysis (Table 2) shows that the values of n in the first three concentrations are in the range of 2,203 to 2,338, indicating that the intensity of adsorption is favorable (n = 1-10), while that at a higher concentration it is considered not favorable because it is higher than 12,036.
Figure 5 graphically demonstrates the applicability of the Freundlich isotherm model. The results obtained from the regression coefficient (R2) are in the range of 0.907 to 0.937, demonstrating that it fits the adsorption isotherm model when applied in linear analysis using the Origin Pro8 V2019 statistical software.
Conclusions
The applicability of the study using cryogel as adsorbent was used for lead removal on the MPAAG-TBA. The adsorption characteristics were brought to different pH and concentration values at a constant temperature in a batch mode process. The adsorption process was using the Langmuir and Fleundlich isotherms through nonlinear and linear analysis using Origin Pro8 software. The equilibration time observed for the MPAAG-TBA was 30 minutes, obtaining its highest removal capacity at pH 5 at a stirring speed of 200 rpm. The experimental data do not fit the Langmuir model at the first two concentrations, however, the Freundlich isotherm model does fit the results. The removal of lead on the MPAAG-TBA turned out to be favorable, therefore it can be concluded that MPAAG-TBA is an efficient adsorbent for the removal of lead from aqueous solutions at low concentrations.
Acknowledgments
Research financed by Research Project Funds 03201502 of Universidad Nacional Autónoma de Nicaragua, Managua (UNAN-Managua).
References
Abdul-Raheim, A. R. M., El-Saeed Shimaa, M., Farag, R. K., & Abdel-Raouf Manar, E. (2016). Low cost biosorbents based on modified starch iron oxide nanocomposites for selective removal of some heavy metals from aqueous solutions. Advanced Materials Letters, 7(5), 402–409. https://doi.org/10.5185/amlett.2016.6061
Alam, Z., Muyibi, S. A., & Toramae, J. (2007). Statistical optimization of adsorption processes for removal of 2,4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. Journal of Environmental Sciences, 19(6), 674–677. https://doi.org/10.1016/S1001-0742(07)60113-2
Ayawei, N., Ebelegi, A. N., & Wankasi, D. (2017). Modelling and Interpretation of Adsorption Isotherms. Journal of Chemistry, 2017. https://doi.org/10.1155/2017/3039817
Badruddoza, A. Z. M., Shawon, Z. B. Z., Tay, W. J. D., Hidajat, K., & Uddin, M. S. (2013). Fe 3O 4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydrate Polymers, 91(1), 322–332. https://doi.org/10.1016/j.carbpol.2012.08.030
Bergmann, C. P. (2015). Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications. https://doi.org/10.1007/978-3-319-18875-1
Boparai, H. K., Joseph, M., & O’Carroll, D. M. (2011). Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. Journal of Hazardous Materials, 186(1), 458–465. https://doi.org/10.1016/j.jhazmat.2010.11.029
Borhade, A. V., & Kale, A. S. (2017). Calcined eggshell as a cost effective material for removal of dyes from aqueous solution. Applied Water Science, 7(8), 4255–4268. https://doi.org/10.1007/s13201-017-0558-9
Bulut, Y., & Baysal, Z. (2006). Removal of Pb(II) from wastewater using wheat bran. Journal of Environmental Management, 78(2), 107–113. https://doi.org/10.1016/j.jenvman.2005.03.010
Carvalho, B. M. A., Da Silva, S. L., Da Silva, L. H. M., Minim, V. P. R., Da Silva, M. C. H., Carvalho, L. M., & Minim, L. A. (2014). Cryogel poly(acrylamide): Synthesis, structure and applications. Separation and Purification Reviews, 43(3), 241–262. https://doi.org/10.1080/15422119.2013.795902
Deng, L., Su, Y., Su, H., Wang, X., & Zhu, X. (2007). Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. Journal of Hazardous Materials, 143(1–2), 220–225. https://doi.org/10.1016/j.jhazmat.2006.09.009
Desta, M. B. (2013). Batch sorption experiments: Langmuir and freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (eragrostis tef) agricultural waste. Journal of Thermodynamics, 1(1). https://doi.org/10.1155/2013/375830
Dubey, S., Gusain, D., & Sharma, Y. C. (2016). Kinetic and isotherm parameter determination for the removal of chromium from aqueous solutions by nanoalumina, a nanoadsorbent. Journal of Molecular Liquids, 219, 1–8. https://doi.org/10.1016/j.molliq.2016.01.021
Eren, E. (2009). Removal of lead ions by Unye (Turkey) bentonite in iron and magnesium oxide-coated forms. Journal of Hazardous Materials, 165(1–3), 63–70. https://doi.org/10.1016/j.jhazmat.2008.09.066
Ertürk, G., & Mattiasson, B. (2014). Cryogels-versatile tools in bioseparation. Journal of Chromatography A, 1357, 24–35. https://doi.org/10.1016/j.chroma.2014.05.055
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156(1), 2–10. https://doi.org/10.1016/j.cej.2009.09.013
Halah, E., Halah, A. El, & López-carrasquero, F. (2018). Applications of hydrogels in the adsorption of metallic ions Aplicación de hidrogeles in la adsorción de iones metálicos. Ciencia e Ingeniería, 39(1), 57–70.
Huang, D. L., Zeng, G. M., Jiang, X. Y., Feng, C. L., Yu, H. Y., Huang, G. H., & Liu, H. L. (2006). Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw. Journal of Hazardous Materials, 134(1–3), 268–276. https://doi.org/10.1016/j.jhazmat.2005.11.021
Onnby, L., Giorgi, C., Plieva, F. M., & Mattiasson, B. (2010). Removal of heavy metals from water effluents using supermacroporous metal chelating cryogels. Biotechnology Progress, 26(5), 1295–1302. https://doi.org/10.1002/btpr.422
Özcan, A. S., Gök, Ö., & Özcan, A. (2009). Adsorption of lead(II) ions onto 8-hydroxy quinoline-immobilized bentonite. Journal of Hazardous Materials, 161(1), 499–509. https://doi.org/10.1016/j.jhazmat.2008.04.002
Pliego-Arreaga, R., Regalado, C., Amaro-Reyes, A., & García-Almendárez, B. E. (2013). Revista Mexicana de Ingeniería Química. Revista Mexicana de Ingeniería Química, 12(3), 505–511. Retrieved from http://www.redalyc.org/articulo.oa?id=62029966013
Plieva, F. M., Karlsson, M., Aguilar, M. R., Gomez, D., Mikhalovsky, S., & Galaev’, I. Y. (2005). Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter, 1(4), 303–309. https://doi.org/10.1039/b510010k
Pourjavadi, A., Abedin-Moghanaki, A., & Hosseini, S. H. (2016). Synthesis of poly(amidoamine)-graft-poly(methyl acrylate) magnetic nanocomposite for removal of lead contaminant from aqueous media. International Journal of Environmental Science and Technology, 13(10), 2437–2448. https://doi.org/10.1007/s13762-016-1063-7
Sari, A., Tuzen, M., Citak, D., & Soylak, M. (2007). Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. Journal of Hazardous Materials, 149(2), 283–291. https://doi.org/10.1016/j.jhazmat.2007.03.078
Sofia, M., & Duarte, N. (2012). Integrated Master in Chemical Engineering Cryogel composites for cadmium removal: evaluating combinations and adsorption by molecularly imprinted polymers.
Tejada-Tovar, C., Villabona-Ortiz, Á., & Garcés-Jaraba, L. (2015). Adsorción de metales pesados en aguas residuales usando materiales de origen biológico. TecnoLógicas, 18(34), 109. https://doi.org/10.22430/22565337.209
Zdravkovic, A., Nikolic, L., Ilic-Stojanovic, S., & Nikolic, V. (2017). The application of hydrogels based on N-isopropylacrylamide and anionic comonomers. Advanced Technologies, 6(1), 33–44. https://doi.org/10.5937/savteh1701033z